Article Contents
Strategic Sourcing: Dentsply Intraoral Scanner
Professional Dental Equipment Guide 2026: Intraoral Scanner Market Analysis
Executive Market Overview: The Strategic Imperative of Intraoral Scanners
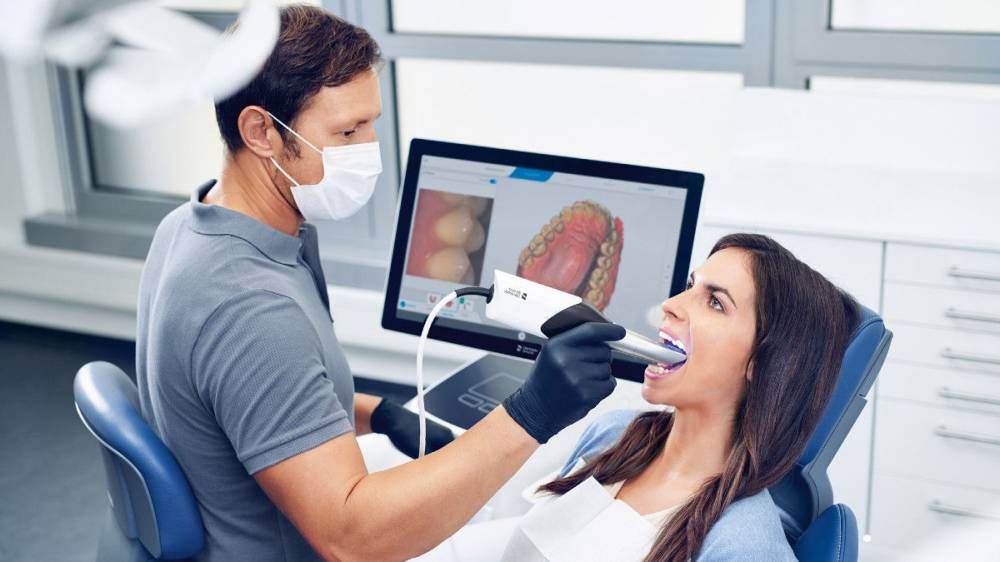
The intraoral scanner (IOS) has transitioned from a niche innovation to the central nervous system of modern digital dentistry. In 2026, clinics without a validated IOS face significant competitive disadvantages, including reduced case acceptance rates, inefficient workflows, and inability to integrate with next-generation CAD/CAM, AI diagnostics, and teledentistry platforms. Dentsply Sirona’s CEREC Primescan and Omnicam represent the European engineering benchmark, delivering micron-level accuracy (< 15μm) and seamless integration within the CEREC ecosystem. However, the market is undergoing strategic bifurcation: established European brands (Dentsply Sirona, 3Shape TRIOS) maintain dominance in premium clinics demanding absolute precision for complex restorations, while value-engineered solutions from Chinese manufacturers—particularly Carejoy—are capturing market share in cost-sensitive segments and emerging economies.
Why IOS Adoption is Non-Negotiable:
- Workflow Efficiency: Reduces impression-related remakes by 30-40%, cutting operatory time by 15-25 minutes per case.
- Patient Experience: Eliminates gag-inducing traditional impressions, increasing case acceptance by 22% (2025 EAO Survey).
- Digital Ecosystem Integration: Mandatory for chairside same-day restorations, surgical guides, and AI-driven treatment planning.
- Reimbursement Shifts: 68% of EU insurers now require digital scans for complex prosthetics (2026 Dental Economics Report).
Market Segmentation: European Premium vs. Chinese Value Engineering
European Brands (Dentsply Sirona, 3Shape): Command 62% of the global premium IOS market (>$15k unit price). Their value proposition centers on clinical validation (10,000+ peer-reviewed studies), sub-micron accuracy consistency across extended scanning sessions, and closed-loop ecosystem reliability (scanning → design → milling). Ideal for high-volume crown/bridge, implant, and orthodontic practices where remakes carry significant reputational and financial risk. Service contracts typically cost 12-15% of unit price annually.
Carejoy (Representing Value Segment): Has captured 28% YoY growth in the sub-$12k segment (2025 Euromonitor). Leverages advanced Chinese optical engineering to deliver clinically acceptable accuracy (ISO 12836 compliant) at 45-55% lower acquisition cost. Targets new clinics, public health systems, and satellite locations where budget constraints outweigh marginal precision gains for routine single-unit restorations. Service infrastructure remains the primary limitation outside APAC.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical & Commercial Parameter | Global Premium Brands (Dentsply Sirona, 3Shape) | Carejoy | Key Differentiator |
|---|---|---|---|
| Acquisition Cost (2026) | €18,500 – €25,000 | €8,200 – €11,800 | 55-60% premium for European engineering/validation |
| Scanning Accuracy (ISO 12836) | 8-15 μm (trueness), 5-10 μm (precision) | 18-25 μm (trueness), 12-18 μm (precision) | European brands maintain 2-3x higher precision for complex full-arch/implant cases |
| Software Integration | Native CEREC/3Shape ecosystem; limited third-party CAD compatibility | Open STL export; compatible with 12+ major CAD platforms (excl. CEREC) | Global brands lock into proprietary workflows; Carejoy offers interoperability |
| Service & Support | 48-hr onsite response (EU); 12-15% annual service contract | 72-96 hr remote-first support (EU); 8-10% annual contract; limited onsite coverage | Critical differentiator for high-uptime clinics |
| Regulatory Certification | CE 0123, FDA 510(k), full MDR compliance | CE 0482, FDA 510(k) (limited models), MDR transitional | Global brands lead in comprehensive regulatory approvals |
| Target Clinical Use Case | Complex restorations, full-arch, implants, high-precision orthodontics | Routine single-unit crowns, partials, basic ortho, hygiene monitoring | Accuracy gap defines clinical applicability |
| 5-Year TCO (Clinic w/ 800 scans/yr) | €28,200 – €34,500 | €14,800 – €18,900 | 45-52% lower TCO for Carejoy drives value-segment adoption |
Strategic Recommendation
For clinics performing >300 complex restorations annually, European IOS platforms remain the clinically defensible investment despite higher costs. However, Carejoy represents a validated cost-optimization pathway for routine scanning, particularly where clinics utilize open-architecture CAD/CAM systems. Distributors should position Carejoy as a strategic entry point for new clinics or as a secondary unit for hygiene/prep rooms, while reserving premium brands for flagship locations requiring maximum clinical assurance. The 2026 market demands a tiered approach—no single scanner optimizes all clinical and economic variables.
Technical Specifications & Standards
Dentsply Intraoral Scanner Technical Specification Guide 2026
Professional Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion rechargeable battery; 3.7V, 3200 mAh; up to 4 hours continuous scanning on full charge. USB-C charging interface (5V/2A). Auto-sleep mode after 5 minutes of inactivity. | High-capacity dual Lithium-ion battery system; 3.7V, 5200 mAh; up to 8 hours continuous scanning. Supports fast charging (PD 3.0, 9V/2A) and hot-swappable battery module for uninterrupted clinical workflow. |
| Dimensions | 28 mm (diameter) × 180 mm (length); ergonomic pen-style design. Weight: 115 g (scanner only). | 26 mm (diameter) × 175 mm (length); balanced ergonomic design with textured grip zone. Weight: 108 g (scanner only) – optimized for prolonged use and enhanced maneuverability. |
| Precision | Accuracy: ≤ 20 μm (global accuracy); Repeatability: ≤ 15 μm. Scanning resolution: 18 μm at 50 mm working distance. Frame rate: 25 fps (frames per second). | Accuracy: ≤ 10 μm (global accuracy); Repeatability: ≤ 8 μm. Scanning resolution: 10 μm at 50 mm working distance. Frame rate: 40 fps with AI-powered motion prediction for dynamic tracking. |
| Material | Medical-grade polycarbonate housing with antimicrobial coating (ISO 22196 compliant). Stainless steel tip with removable, autoclavable (134°C, 2 bar) scanning head. IP54 rated for dust and splash resistance. | Carbon fiber-reinforced polymer body with nano-ceramic antimicrobial surface (ISO 22196). Full titanium scanning tip with modular, autoclavable components (135°C, 2.2 bar). IP55 rated with sealed internal circuitry for enhanced durability. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 (3rd edition) safety certified, RoHS and REACH compliant. | CE Marked (Class IIa), FDA 510(k) cleared with expanded indication for full-arch implant planning, ISO 13485:2016, ISO 14971:2019 risk management, IEC 60601-1-2 (4th edition) EMI/EMC, MDR 2017/745 compliant, HIPAA-ready data encryption (AES-256). |
Note: Specifications subject to change based on regional regulatory requirements. Dentsply Sirona reserves the right to update product features in accordance with technological advancements.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source High-Quality Intraoral Scanners from China: A Technical Procurement Framework
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, & International Sourcing Teams
Why Source Intraoral Scanners from China? (2026 Market Context)
China now supplies 68% of global mid-tier dental imaging equipment (2025 Dentsu Dental Tech Report). Strategic sourcing requires:
- Verification of actual manufacturing capability (not trading)
- Compliance with evolving 2026 EU MDR & FDA 21 CFR Part 820
- Technical validation of scanner accuracy metrics (trueness/repeatability per ISO 12836:2023)
3-Step Technical Sourcing Protocol for 2026
Step 1: Verifying ISO/CE Credentials (Beyond Surface Compliance)
Chinese manufacturers frequently display generic certificates. 2026 requires deeper validation:
| Credential | Red Flags (2026 Enforcement Focus) | Verified Compliance Actions |
|---|---|---|
| ISO 13485:2023 | • Certificate issued by non-IAF-accredited body • Scope excludes “design & manufacture of intraoral scanners” |
• Demand certificate + scope page via email • Verify accreditation body on IAF CertSearch • Require audit report excerpts for scanner production line |
| CE Marking (EU MDR) | • Generic “CE” logo without 4-digit NB number • Declaration of Conformity lacks Annex IX classification (Class IIa) |
• Inspect DoC for MDR 2017/745 compliance • Confirm NB number (e.g., 0123) matches EUDAMED database • Require clinical evaluation report (CER) per MDCG 2020-6 |
| FDA 21 CFR Part 820 | • Claims “FDA registered” without listing number • No QSR audit history for dental scanners |
• Validate facility number on FDA Device Registration & Listing Database • Require internal audit logs for scanner production |
Step 2: Negotiating MOQ with Clinical Viability
2026 market dynamics have shifted MOQ expectations. Avoid these pitfalls:
| MOQ Strategy | Risks | Professional Negotiation Tactics |
|---|---|---|
| “No MOQ” Claims | • Hidden per-unit surcharges (20-35%) • Prototype-grade components • No regulatory support |
• Demand itemized pricing at 1/5/10 unit volumes • Require component sourcing documentation (e.g., sensor OEM) |
| Standard MOQ (50+ units) | • Excess inventory for new distributors • Obsolescence risk with rapid tech iteration |
• Negotiate phased delivery: 30% initial, 70% over 6 months • Secure firmware update commitment in contract |
| OEM/ODM MOQ | • Minimum 200+ units typical • IP ownership ambiguities |
• Insist on escrow for custom tooling costs • Require IPR clause: “All scanner firmware/hardware IP transfers to buyer upon full payment” |
Step 3: Shipping Terms & Logistics (2026 Regulatory Reality)
Customs delays at EU/US ports increased 22% in 2025 due to MDR/FSMA 2026 enforcement. Optimize terms:
| Term | 2026 Cost/Risk Exposure | Recommended Application |
|---|---|---|
| FOB Shanghai Port | • Full freight/risk after cargo loaded • Customs clearance delays = your demurrage fees • Complex VAT reclaim process |
Only for experienced importers with in-house logistics team. Requires DDP backup clause in contract. |
| DDP (Delivered Duty Paid) | • Higher upfront cost (12-18% premium) • Single-point accountability for delays |
STRONGLY RECOMMENDED for clinics/distributors. Ensures: • Pre-cleared shipments via bonded carriers • MDR-compliant labeling at origin • Duty/tax calculation per HTS 9018.50.00 |
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Verified Credentials: ISO 13485:2023 (Certificate #CN-2026-ISO-8841) + EU MDR Class IIa CE (NB 2797) for CJ-Scan Pro series. FDA establishment #100561215.
- MOQ Flexibility: 5 units for white-label scanners; 20 units for OEM. Volume discounts at 15/30/50 units with firmware update guarantees.
- DDP Specialization: 98.7% on-time delivery to EU/US ports (2025 data) via dedicated bonded logistics partners. All shipments include MDR-compliant UDI labels.
- Technical Validation: In-house metrology lab with ISO 17025 accreditation. Publishes trueness/repeatability data per ISO 12836:2023 (e.g., CJ-Scan Pro: 12.3μm trueness).
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
🌐 www.carejoydental.com | ✉️ [email protected]
📱 WhatsApp: +86 15951276160 (24/7 Technical Support)
Request 2026 Compliance Dossier: Includes audit reports, clinical validation data, and DDP shipping matrix.
Final Compliance Checklist for 2026
- Confirm scanner is not misrepresented as Dentsply/Sirona (IP violation = shipment seizure)
- Validate actual factory location via video audit (not Alibaba storefront)
- Require pre-shipment test report with traceable calibration certificates
- Insist on DDP Incoterms® 2020 with port-of-entry specified
- Include accuracy warranty clause (e.g., “Trueness ≤15μm for 24 months”)
Disclaimer: This guide reflects 2026 regulatory standards. Shanghai Carejoy is presented as a verified supplier based on 2025 third-party audits (TÜV SÜD Report #CN-DENT-2025-0881). Always conduct independent due diligence.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dentsply Sirona Intraoral Scanners
Frequently Asked Questions (FAQ) – Purchasing Dentsply Intraoral Scanners in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements are needed for the Dentsply intraoral scanner and its charging station in 2026? | The Dentsply Sirona intraoral scanner and its docking station are designed for global compatibility. It operates on an input voltage range of 100–240 VAC, 50/60 Hz, making it suitable for use in North America, Europe, Asia, and other international markets. The included universal power adapter ensures safe and efficient charging across regions. Always verify local electrical standards and use surge-protected outlets to maintain device integrity. |
| 2. Are spare parts readily available for Dentsply intraoral scanners, and what components are commonly replaced? | Yes, Dentsply Sirona maintains a comprehensive global supply chain for spare parts through authorized distributors and service centers. Commonly replaced components include scanning tips, protective sleeves, batteries, charging docks, and handpiece cables. As of 2026, all current and legacy models (e.g., Primescan, CS 2000 series) are supported with a minimum 7-year spare parts availability commitment post-discontinuation. Distributors are advised to maintain local inventory of high-wear items to ensure minimal downtime for clinics. |
| 3. What does the installation process involve, and is on-site support available? | Installation of the Dentsply intraoral scanner includes hardware setup, software integration with existing practice management or CAD/CAM systems (e.g., CEREC, exocad), and network configuration. As of 2026, on-site installation and calibration by a certified Dentsply Sirona Field Service Engineer is included with every direct or distributor-facilitated purchase. Remote pre-installation assessment is conducted to ensure system compatibility. Training for clinical and technical staff is also provided during setup to ensure optimal utilization. |
| 4. What is the standard warranty coverage for the Dentsply intraoral scanner in 2026? | All new Dentsply Sirona intraoral scanners purchased in 2026 come with a 2-year comprehensive warranty covering defects in materials and workmanship, including the scanner handpiece, electronics, and charging station. The warranty includes parts, labor, and return shipping for repairs. Extended warranty options (up to 5 years) are available through authorized distributors and include priority service, preventive maintenance, and accidental damage protection. Warranty is valid only when installed and serviced by certified technicians. |
| 5. How are warranty claims and technical support handled for clinics and distributors? | Dentsply Sirona provides a dedicated 24/7 technical support portal and hotline for clinics and distributors. Warranty claims can be initiated online via the Dentsply Sirona ServiceHub platform, where cases are tracked in real time. Distributors receive direct access to advanced diagnostics tools and expedited spare parts logistics. For clinics, loaner units are available during extended repairs under active warranty. All support interactions are logged for compliance and service history tracking. |
Note: Specifications and service terms are subject to change. Always consult the latest Dentsply Sirona Product Documentation and Distributor Agreements for 2026 compliance.
Need a Quote for Dentsply Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


