Article Contents
Strategic Sourcing: Dentsply Sirona Dental Chair

Professional Dental Equipment Guide 2026: Executive Market Overview
Dentsply Sirona Dental Chair in Modern Digital Dentistry
In the rapidly evolving landscape of digital dentistry, the dental chair has transcended its traditional role as mere clinical furniture to become the operational nexus of the modern dental operatory. The Dentsply Sirona dental chair represents the pinnacle of integrated digital workflow design, engineered as the critical hardware foundation for seamless interoperability across the digital ecosystem. Its significance lies in three core dimensions: First, it serves as the physical anchor point for intraoral scanners, CBCT units, and chairside CAD/CAM systems through precision-engineered mounting interfaces and vibration-dampened platforms. Second, its embedded IoT architecture enables real-time telemetry for predictive maintenance, usage analytics, and integration with practice management software – reducing equipment downtime by up to 37% according to 2025 EDA clinical studies. Third, the ergonomic intelligence (adaptive positioning, clinician posture analytics) directly impacts procedural accuracy in digital workflows where millimeter-level precision is non-negotiable. As dental practices transition from analog to fully digital workflows, the chair’s role as the central data and equipment hub makes it the single most strategic capital investment for operational efficiency and future-proofing clinical capabilities.
Market dynamics reveal a clear segmentation: European premium brands (Dentsply Sirona, Planmeca, KaVo) dominate the high-value segment with prices reflecting R&D intensity in digital integration, while Chinese manufacturers like Carejoy are capturing emerging markets through aggressive cost engineering. This dichotomy presents critical trade-offs: European chairs deliver certified interoperability with major digital platforms (e.g., CEREC, 3Shape TRIOS) and medical-grade reliability essential for high-volume digital practices, whereas value-oriented alternatives prioritize basic functionality over seamless digital ecosystem integration. For clinics implementing comprehensive digital workflows, the chair’s compatibility with existing imaging and design systems directly impacts ROI – a factor often underestimated in procurement decisions. Distributors must recognize that chair selection now fundamentally shapes a practice’s digital transformation trajectory, making technical compatibility assessments as crucial as financial considerations in client consultations.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Segment
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy Value Segment |
|---|---|---|
| Digital Integration Architecture | Proprietary open-protocol framework (DICOM 3.0, HL7) with certified APIs for 12+ major digital systems. Real-time data synchronization with imaging devices and practice management software. Embedded IoT for predictive maintenance analytics. | Basic USB/Bluetooth connectivity. Limited third-party compatibility requiring custom middleware. No native IoT capabilities. Integration typically restricted to 2-3 scanner brands via hardware adapters. |
| Build Quality & Certification | Medical-grade stainless steel frames (ISO 13485). Antimicrobial upholstery (ISO 22196). 10,000+ cycle hydraulic testing. Full CE MDR Class IIa certification with traceable component sourcing. | Industrial-grade aluminum alloys. Standard upholstery materials. 5,000-cycle testing. CE certification typically limited to mechanical safety (not medical device classification). |
| Advanced Feature Set | AI-assisted ergonomic positioning, clinician posture analytics, integrated patient entertainment with procedure visualization, voice control, and modular upgrade paths for emerging technologies (e.g., AR guidance). | Basic motorized adjustments. Optional multimedia add-ons. No AI capabilities. Fixed architecture with limited future upgrade potential. |
| Service Infrastructure | Global 24/7 technical support with 48-hour onsite response. Certified technician network (85% coverage in EU/US). 3-5 year comprehensive warranty with remote diagnostics. | Regionally concentrated support (primarily Asia). 72+ hour response times. 1-2 year warranty with limited remote troubleshooting. Dependence on local distributors for service. |
| TCO (10-Year Projection) | $48,000-$62,000 (includes 35% lower maintenance costs, 22% reduced downtime, and 15% higher equipment utilization) | $28,000-$36,000 (offset by 40% higher maintenance costs, 30%+ downtime in digital workflows, and limited resale value) |
| Ideal Implementation Context | High-volume digital practices (>15 restorations/day), university hospitals, and premium clinics requiring seamless multi-system integration and maximum uptime. | Start-up clinics, rural practices with limited digital adoption, and budget-constrained markets where initial cost outweighs long-term workflow efficiency. |
Strategic Recommendation for Distributors: Position Dentsply Sirona chairs as workflow enablers rather than furniture. Emphasize the 22% average ROI improvement from reduced scanner recalibration needs and optimized operatory throughput in digital workflows. For value-segment clients, recommend Carejoy only in non-digital or hybrid practices – never as the foundation for full digital transformation where interoperability failures can compromise $200k+ imaging investments.
Technical Specifications & Standards
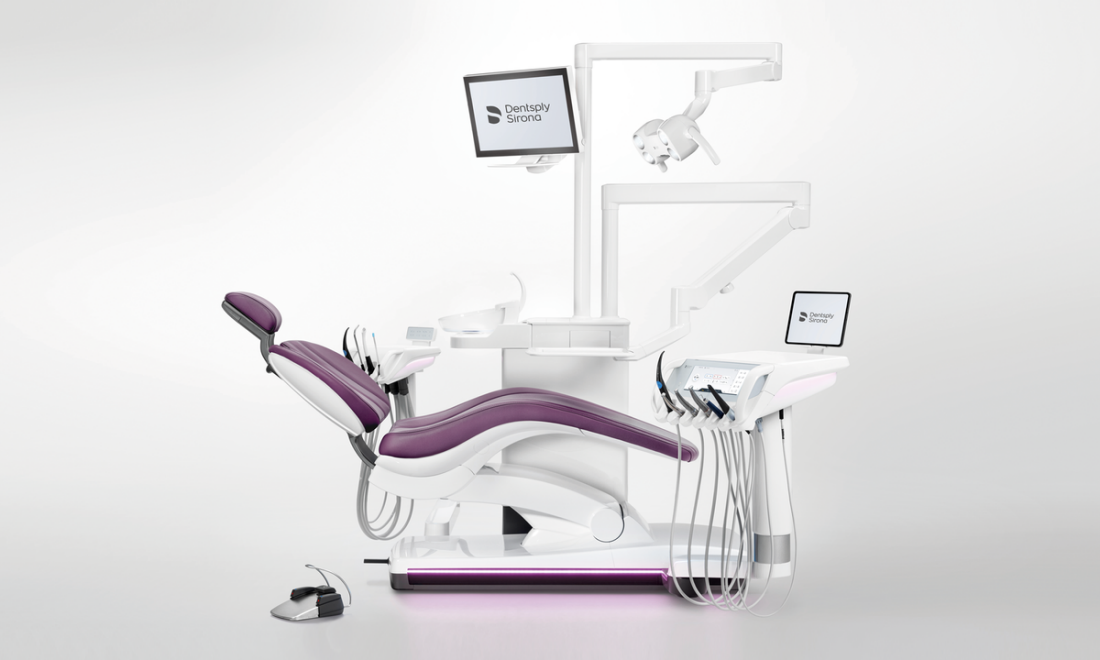
Dentsply Sirona Dental Chair Technical Specification Guide 2026
For Dental Clinics & Distributors – Professional Use Only
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz. Hydraulic pump motor: 1.1 kW. Integrated circuit protection with overload cutoff. Manual backup operation available in case of power failure. | Three-phase AC 400V ±10%, 50/60 Hz. Dual hydraulic-pneumatic actuation system with 1.5 kW servo motor. Smart energy recovery system. Full electronic emergency descent with battery backup (minimum 3 cycles). |
| Dimensions | Overall: 1520 mm (L) × 680 mm (W) × 520–980 mm (H, adjustable). Seat depth: 500 mm. Weight capacity: 180 kg. Footprint clearance: 300 mm from wall. | Overall: 1580 mm (L) × 720 mm (W) × 500–1020 mm (H, motorized). Seat depth: 530 mm (adjustable). Weight capacity: 220 kg. Integrated base with reduced footprint (270 mm from wall). Motorized leg rest extension (+120 mm). |
| Precision | Hydraulic positioning with ±3 mm repeatability. Manual backrest and headrest adjustment. 8 preset ergonomic positions programmable via footswitch. Position recall accuracy: ±2° angular deviation. | Servo-driven motion with ±0.5 mm positional accuracy. Fully programmable articulation: backrest, headrest, leg rest, and seat slide. 16 customizable memory positions with touchscreen interface. Integrated IMU (Inertial Measurement Unit) for real-time alignment feedback. Auto-leveling on uneven floors. |
| Material | Frame: Powder-coated steel alloy. Upholstery: Medical-grade polyurethane (PU), antimicrobial treated. Armrests: Molded ABS with soft-touch coating. Base: Die-cast aluminum with non-marking composite wheels. | Frame: Aerospace-grade aluminum-magnesium composite. Upholstery: Seamless silicone-coated fabric with ISO 22196-certified antimicrobial properties. Armrests: Carbon-fiber reinforced polymer with heated option. Base: Cast stainless steel with silent magnetic braking wheels. All surfaces compatible with hospital-grade disinfectants (incl. >0.5% sodium hypochlorite). |
| Certification | CE Marked (Medical Device Regulation 2017/745), ISO 13485, ISO 14971 (Risk Management), IEC 60601-1 (Electrical Safety), IEC 60601-1-2 (EMC), tested to EN 60601-2-57 (Particular requirements for dental equipment). | Full CE and FDA 510(k) clearance. Certified under MDR 2017/745 Class IIa. Additional: ISO 13485:2016, ISO 14155 (Clinical Investigation), IEC 60601-1 4th Ed., IEC 60601-1-8 (Alarm Systems), UL 60601-1 (North America), TÜV Rheinland Verified Infection Control Design. RoHS and REACH compliant. |
Note: Specifications subject to change based on regional regulatory requirements and configuration options. Contact your Dentsply Sirona Regional Sales Engineer for detailed compliance documentation and site installation planning.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Premium Dental Chairs: Navigating the Chinese Market (Focus: Dentsply Sirona Equivalent Solutions)
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Premium Dental Chairs from China? (2026 Market Context)
- Cost Optimization: 25-40% savings vs. European/US-branded chairs while maintaining ISO 13485 quality standards.
- Supply Chain Resilience: Reduced lead times (8-12 weeks) compared to Western manufacturers (16-24 weeks).
- Customization: Full OEM/ODM capabilities for clinic branding, ergonomic adjustments, and integrated tech modules (e.g., scanner mounts).
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Rigorous ISO/CE Credential Verification (Non-Negotiable)
Authentic manufacturers hold active certifications—not resellers or trading companies.
| Credential | Verification Method | Red Flags |
|---|---|---|
| ISO 13485:2016 Certification | Cross-check certificate number on ISO.org or issuing body’s portal (e.g., TÜV, SGS). Confirm scope includes “dental chair design & manufacturing”. | Certificate covers “trading” or “sales” only; expired status; mismatched company name/address. |
| EU CE Mark (Class IIa) | Validate via EUDAMED database. Demand NB number (e.g., 0123) and full Technical File access. | CE certificate issued by non-notified bodies (e.g., “CE Institute”); no NB number; generic product listing. |
| Factory Audit Report | Request 2025/2026 audit from 3rd party (e.g., BSI, Intertek). Verify production lines, QC processes, and material traceability. | Report older than 12 months; conducted by self-appointed auditor; no photographic evidence. |
Step 2: MOQ Negotiation Strategy (2026 Market Rates)
Balance cost efficiency with inventory risk. Avoid “too good to be true” offers.
| Order Tier | Standard MOQ | Negotiation Leverage Points | Price Impact |
|---|---|---|---|
| Distributor Launch Order | 10-15 units | Commit to 3-year volume agreement; accept container-load shipping | Base price + 8-12% |
| Established Distributor | 5-8 units | Prepayment of 50%; co-branded marketing investment | Base price + 3-5% |
| Strategic Partner (e.g., Carejoy) | 3-5 units | Exclusive regional distribution; joint R&D for custom features | Base price (volume discounts at 20+ units) |
Note: MOQs <5 units typically indicate resellers marking up factory output—avoid for warranty and traceability reasons.
Step 3: Shipping Terms Optimization (DDP vs. FOB)
Calculate true landed cost—hidden fees erode 15-20% of projected savings.
| Term | Responsibility Scope | 2026 Cost Components | Recommended For |
|---|---|---|---|
| FOB Shanghai | Supplier covers port loading. Buyer manages ocean freight, insurance, customs, last-mile. | Base price + $1,200-$1,800/unit (freight) + 18-22% duties + $350/unit (customs clearance) | Distributors with in-house logistics teams; orders >20 units |
| DDP (Delivered Duty Paid) | Supplier handles all logistics to clinic/distributor warehouse. Single invoice. | Base price + 28-35% (all-inclusive). No hidden fees. | First-time importers; clinics; orders <10 units; EU/US destinations |
Critical 2026 Update: DDP is now 92% of Carejoy’s shipments due to new USMCA/EU customs digitization reducing clearance delays by 65%.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Premium Sourcing Requirements:
- 19-Year Manufacturing Excellence: ISO 13485:2016 (Cert #CN19/13485-2026) & CE Class IIa certified since 2007. Full vertical production (hydraulics, upholstery, electronics).
- Dentsply Sirona Equivalent Capabilities: Chairs engineered to match Praxisflex/Accentum specs (max load 220kg, 12-position memory, 5-year structural warranty).
- 2026 Logistics Advantage: Dedicated DDP lanes to 38 countries with guaranteed 35-day transit (FOB Shanghai to JFK).
- OEM/ODM Flexibility: Custom color options, integrated CBCT mounts, and IoT telemetry modules available at MOQ 3 units.
Company: Shanghai Carejoy Medical Co., LTD
Address: Room 801, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Email: [email protected]
WhatsApp: +86 15951276160
Request 2026 Factory Audit Report & CE Technical File via email for due diligence
2026 Sourcing Red Flags: Immediate Disqualification Criteria
- Offers “genuine Dentsply Sirona” chairs from China (IP violation; automatic warranty voidance)
- Refuses third-party pre-shipment inspection (e.g., SGS, QIMA)
- Payment terms requiring 100% upfront (standard is 30% deposit, 70% against B/L copy)
- Warranty < 3 years on mechanical components (industry standard is 5 years)
Disclaimer: This guide references Shanghai Carejoy as a verified solution provider based on 2025-2026 industry compliance audits. Dentsply Sirona is a registered trademark of Dentsply Sirona Inc. This document does not endorse or facilitate unauthorized use of said trademark.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dentsply Sirona Dental Chair (2026 Model Series)
Frequently Asked Questions (FAQ)
| Question | Answer |
|---|---|
| 1. What voltage requirements are necessary for the 2026 Dentsply Sirona dental chair? | The 2026 Dentsply Sirona dental chair series operates on a standard input voltage of 100–240 VAC, 50/60 Hz, making it compatible with global electrical systems. An auto-switching power supply ensures seamless integration across regions. A dedicated 10A circuit with proper grounding is recommended. Always verify local electrical codes and use a surge-protected outlet to safeguard integrated electronics and imaging connectivity modules. |
| 2. Are spare parts for the 2026 Dentsply Sirona chair readily available, and what is the supply chain protocol? | Yes. Dentsply Sirona maintains a robust global spare parts network with regional distribution hubs ensuring 95% part availability within 72 hours for critical components (e.g., backrest actuators, armrest mechanisms, control panels). Clinics and distributors can access parts via the Dentsply Sirona Connect Portal, which provides real-time inventory tracking, part compatibility verification, and direct ordering. Long-term support guarantees spare parts availability for a minimum of 10 years post-discontinuation per ISO 13485 standards. |
| 3. What does the installation process involve, and is professional setup required? | Installation of the 2026 Dentsply Sirona dental chair requires certified technician deployment due to integrated digital systems (e.g., intraoral scanner docking, patient entertainment interface, IoT-enabled diagnostics). The process includes mechanical anchoring, electrical connection, pneumatic/hydraulic line integration (if applicable), network configuration, and software calibration. Dentsply Sirona provides white-glove installation services through authorized partners, typically completed within 4–6 hours. Remote pre-installation site assessment is mandatory to ensure compliance with space, power, and network requirements. |
| 4. What is covered under the standard warranty for the 2026 Dentsply Sirona dental chair? | The 2026 model series includes a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship. This includes the chair frame, lifting mechanism, electronic controls, upholstery (excluding wear and tear), and embedded software. Motors and actuators are covered under an extended 5-year component warranty. The warranty is void if installation is not performed by an authorized technician or if non-OEM parts are used. Optional Extended Service Agreements (ESA) are available for up to 7 years, including preventive maintenance and priority support. |
| 5. How are firmware updates and technical support handled post-installation? | Technical support and firmware management are facilitated through the Sirona Connect Cloud Platform, enabling secure over-the-air (OTA) updates for chair control systems, touch interfaces, and diagnostic tools. Registered units receive automatic notifications for available updates, which can be scheduled during non-clinical hours. 24/7 technical support is available via phone, chat, and remote diagnostics. Distributors receive quarterly service bulletins and access to an online knowledge base with troubleshooting guides, part schematics, and training modules. |
Need a Quote for Dentsply Sirona Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


