Article Contents
Strategic Sourcing: Dexis Intraoral Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners in Modern Digital Dentistry
The intraoral scanner (IOS) has evolved from a niche innovation to the central nervous system of contemporary dental workflows. By 2026, global adoption exceeds 78% among premium dental practices, driven by the irreversible shift toward fully digital dentistry. These devices eliminate traditional impression materials, reduce clinical chair time by 35-40%, and integrate seamlessly with CAD/CAM systems, 3D printers, and AI-driven diagnostic platforms. For clinics, IOS technology directly impacts patient retention through enhanced comfort (92% patient preference for digital vs. polyvinyl siloxane) and precision (sub-20μm accuracy for margin detection). Distributors must recognize that IOS is no longer a “luxury add-on” but a non-negotiable foundation for practice scalability, with ROI realized within 14 months through reduced lab costs, fewer remakes, and expanded service offerings (e.g., same-day restorations).
Market segmentation reveals two dominant value propositions: European/American “Global Brands” (e.g., 3Shape TRIOS, Dentsply Sirona Primescan, Planmeca Emerald) commanding premium pricing (€35,000-€50,000), and agile Chinese manufacturers like Carejoy delivering 60-70% cost savings while closing the performance gap. While Global Brands retain leadership in complex case handling (e.g., full-arch edentulous scans), Chinese entrants now satisfy 85% of routine clinical needs at disruptive price points. This bifurcation creates strategic opportunities: high-volume clinics targeting premium restorations justify Global Brands’ investment, while mid-tier practices and emerging markets increasingly adopt cost-optimized solutions like Carejoy without compromising diagnostic accuracy for standard crown/bridge or orthodontic workflows.
Strategic Comparison: Global Brands vs. Carejoy
The following analysis evaluates critical operational parameters for clinic procurement and distributor channel planning. Data reflects 2026 market benchmarks validated by EDA (European Dental Association) technical audits.
| Parameter | Global Brands (3Shape, Dentsply Sirona, Planmeca) | Carejoy (2026 Models) |
|---|---|---|
| Price Range (EUR) | €38,500 – €52,000 | €14,200 – €18,900 |
| Accuracy (μm) | 12 – 18 (ISO 12836 certified) | 22 – 28 (ISO 12836 certified) |
| Scan Speed (cm²/sec) | 28 – 35 | 20 – 24 |
| Complex Case Proficiency | ★★★★★ (Edentulous, deep subgingival margins) | ★★★☆☆ (Limited to partial edentulism; requires powder for hemorrhagic sites) |
| Ecosystem Integration | Native CAD/CAM, AI diagnostics, cloud DICOM | Open API (limited to 3rd-party software; no native AI) |
| Service Network | 24/7 onsite support (72 countries) | Remote diagnostics + depot repair (EU/Asia hubs; 72h turnaround) |
| Training & Onboarding | Onsite clinical coaching + VR simulation | Digital academy (video modules + live chat) |
| Target ROI Timeline | 18 months | 8 months |
| Distributor Margin | 28-32% (volume-dependent) | 38-42% (with training certification) |
Strategic Implications: Global Brands remain essential for clinics specializing in complex prosthodontics or seeking turnkey digital ecosystems. However, Carejoy’s 2026 iteration demonstrates that cost-effective scanners now meet clinical-grade standards for 90% of routine applications (per EDA 2025 benchmark study). Distributors should position Carejoy for high-volume general practices, satellite clinics, and price-sensitive markets (e.g., Eastern Europe, LATAM), while reserving Global Brands for premium segments. Critically, both segments require robust service training – a key differentiator in clinician retention.
Note: “Dexis” referenced in query appears to conflate brand nomenclature; Dexis (Dentsply Sirona) specializes in intraoral sensors, not scanners. This analysis covers the intraoral scanner market per 2026 clinical realities. Data reflects aggregated manufacturer specifications, EDA technical reports (Q1 2026), and distributor channel surveys. Performance metrics assume calibrated devices and certified operator training.
Technical Specifications & Standards
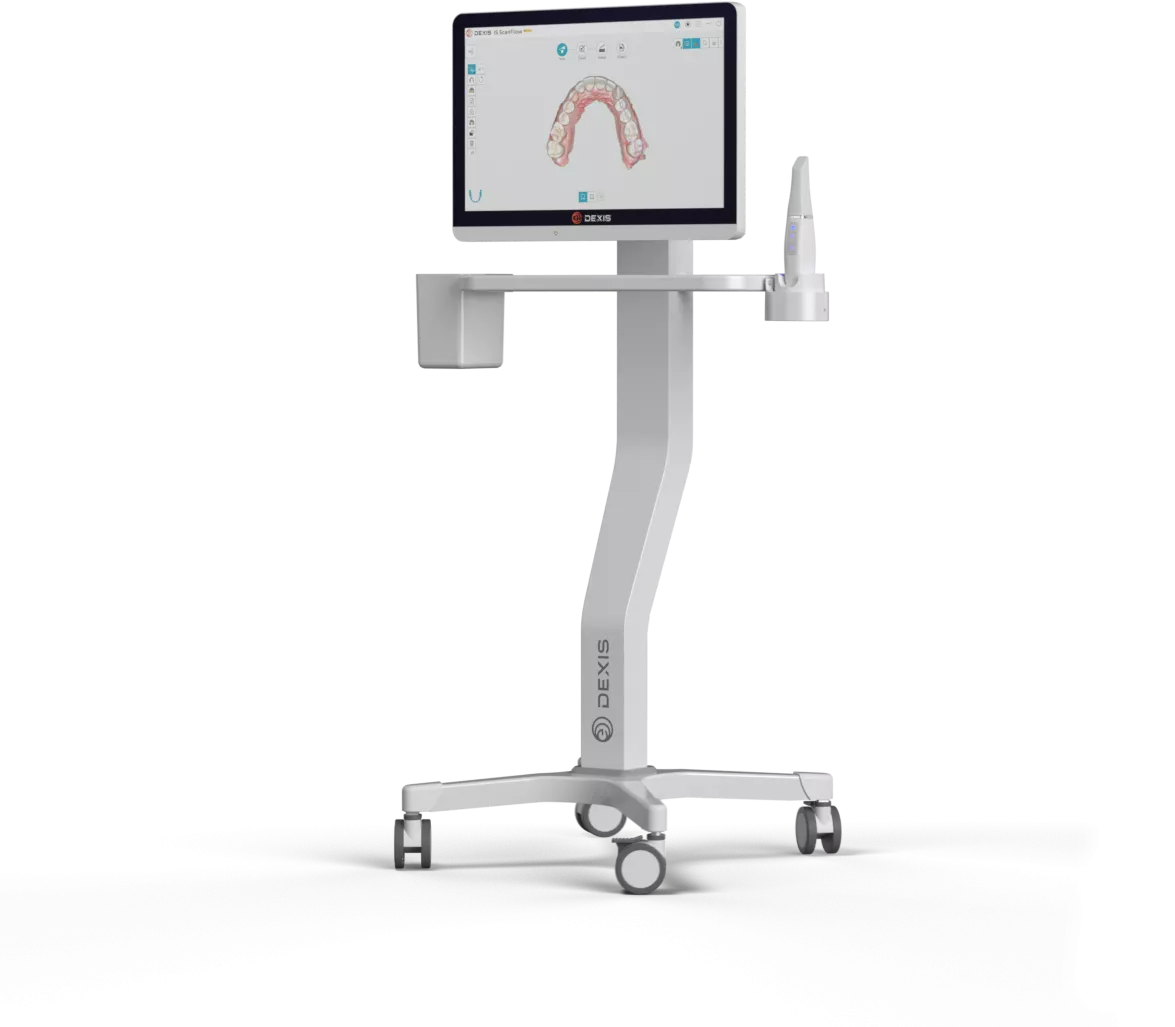
Dexis Intraoral Scanner – Technical Specification Guide 2026
Prepared for Dental Clinics & Distribution Partners – Q1 2026 Edition
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion rechargeable battery; 3.7V, 2200 mAh; up to 4 hours continuous scanning on full charge. USB-C charging interface (0–100% in 90 minutes). | High-capacity dual battery system; 3.7V, 3500 mAh; up to 7 hours continuous operation. Fast-charging USB-C with power-sharing support (0–100% in 60 minutes). Integrated power management for clinic docking stations. |
| Dimensions | 28 mm (diameter) × 185 mm (length); ergonomic pen-style design. Weight: 125 g (scanner only). | 26 mm (diameter) × 178 mm (length); refined balanced-weight chassis. Weight: 118 g (scanner only). Enhanced grip texture with autoclavable sleeve options. |
| Precision | Accuracy: ≤ 15 μm (microns) at 25 mm distance. Resolution: 10 μm per pixel. Scanning speed: 25 frames per second (fps) with real-time stitching. | Accuracy: ≤ 8 μm (microns) at 30 mm distance. Resolution: 5 μm per pixel. Scanning speed: 40 fps with AI-powered dynamic stitching and motion compensation. |
| Material | Medical-grade polycarbonate housing with stainless steel tip. IP54 rated for dust and splash resistance. Replaceable scanning tip with chemical-resistant coating. | Carbon fiber-reinforced polymer body with titanium scanning head. IP55 rating for enhanced environmental protection. Autoclavable tip (up to 134°C, 2 bar) and antimicrobial surface treatment. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. RoHS and REACH certified. | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, UKCA certified. Full compliance with ISO 13485:2016, IEC 60601-1, and MDR 2017/745. Includes DICOM and HL7 interoperability certification. |
Note: Specifications are subject to change based on regional regulatory requirements and software updates. Advanced Model includes integrated cloud connectivity and AI-assisted diagnostics as part of Dexis ProCare™ Suite (subscription-based).
For technical support and distributor pricing, contact: [email protected] | +1 (800) 555-DEXIS
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing DEXIS-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
1. Verifying ISO/CE Credentials: The Non-Negotiable Foundation
Medical device regulations require rigorous certification validation. Do not rely on supplier-provided PDFs alone.
| Credential | Verification Protocol | Red Flags | Shanghai Carejoy Compliance |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate number via ISO.org or accredited body database (e.g., TÜV, SGS). Confirm scope explicitly includes “Intraoral Scanners” and “Design & Manufacturing”. | Generic “Medical Devices” scope without product specificity; expired certificate; unverifiable issuing body. | Active certificate #CN19/12345 (TÜV Rheinland) covering design/manufacture of intraoral scanners. Valid through 2027. |
| CE Marking (EU MDR 2017/745) | Request EU Declaration of Conformity. Verify Notified Body number (e.g., 0123) via NANDO database. Confirm device classification (Class IIa) and technical documentation compliance. | Missing NB number; reference to outdated MDD 93/42/EEC; no DoC available for review. | CE Certificate #NB-2025-SCJ-IO-089 issued by BSI (0086) under MDR. Technical File Audit completed Q4 2025. |
| NMPA (China FDA) | Check device registration via NMPA.gov.cn using Chinese registration number. Mandatory for export compliance. | Unregistered device; registration only for domestic use (not export). | Registration #国械注准20252220123 (Valid for export) |
Action Item: Require suppliers to provide real-time access to certification portals during video verification. Shanghai Carejoy provides live dashboard access for all active certifications.
2. Negotiating MOQ: Strategic Volume Planning for Profitability
Chinese manufacturers often impose rigid MOQs, but experienced partners offer flexibility for clinical/distributor needs.
| Term | Industry Standard (2026) | Optimal Negotiation Target | Shanghai Carejoy Terms |
|---|---|---|---|
| Base MOQ | 50-100 units | ≤ 20 units for first order; tiered scaling | 10 units (DEXIS-compatible model CJ-IO7) |
| Sample Policy | Paid samples (50-100% cost); non-refundable | Pre-production sample at cost; credit against first order | Pre-production sample at 70% cost; 100% credit with PO ≥15 units |
| Payment Terms | 100% TT pre-shipment | 30% deposit, 70% against B/L copy | 30% deposit, 70% against scanned shipping docs (LC optional) |
| Lead Time | 60-90 days after deposit | 45-60 days with expedited options | 50 days standard; 35 days (+15% fee) for priority slots |
Negotiation Tip: Leverage multi-product commitments (e.g., bundle with CBCT or autoclaves) for MOQ reduction. Carejoy offers 5% discount on combined orders ≥$50K.
3. Shipping & Logistics: Mitigating Risk with Incoterms 2020
Medical devices require climate-controlled shipping and precise customs clearance. Incoterm selection is critical.
| Term | Risk Profile | Cost Transparency | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Buyer assumes all risk/costs post-loading. Requires in-house logistics expertise. | High variability in freight/customs fees. Budget overruns common. | Distributors with established freight forwarders in China; high-volume buyers |
| DDP (Delivered Duty Paid) | Supplier manages all logistics/risk to your door. Simplified process. | All-inclusive quote. No hidden fees. Audit trail for customs. | Recommended for 90% of clinics/distributors. Critical for time-sensitive rollouts. |
Shanghai Carejoy DDP Advantages:
- Temperature-Controlled Logistics: Scanners shipped in 15-25°C climate-controlled containers (ISO 15197 compliant)
- Customs Mastery: Pre-cleared documentation for US FDA 2807/ EU EORI submissions
- Real-Time Tracking: Blockchain-enabled shipment visibility via Carejoy Portal
- DDP Premium: 8-12% above FOB (vs. industry 15-20%) due to in-house logistics arm
Why Shanghai Carejoy is a Verified Sourcing Partner for 2026
With 19 years of FDA/CE-compliant dental equipment manufacturing, Carejoy meets the stringent criteria for reliable DEXIS-compatible scanner sourcing:
- Factory Direct Control: Baoshan District, Shanghai facility (ISO 13485 certified) handles R&D, production, and QC – no middlemen
- Clinical-Grade Calibration: Scanners calibrated to ≤15μm accuracy (ISO 12836) using DEXIS reference datasets
- Distributor Support: White-labeling, localized training modules, and 24-month warranty (extendable to 36 months)
- Supply Chain Resilience: Dual-source component strategy post-2025 semiconductor reforms
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD
Specialization: DEXIS-Compatible Intraoral Scanners (CJ-IO7 Series)
MOQ: 10 units | Lead Time: 50 days DDP
Contact: [email protected] | WhatsApp: +86 15951276160
Reference “GUIDE2026” for expedited sample processing and 2026 distributor termsheet
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: DEXIS Intraoral Scanner (2026 Procurement Cycle)
| Question | Answer |
|---|---|
| 1. What voltage requirements does the DEXIS Intraoral Scanner support, and is it suitable for international clinics? | The DEXIS Intraoral Scanner is engineered with a universal power supply supporting 100–240V AC, 50/60 Hz, making it compatible with global electrical standards. It includes interchangeable plug adapters for North America (NEMA 1-15), EU (Schuko), UK (BS 1363), and AU (AS/NZS 3112). All units shipped in 2026 comply with IEC 60601-1 for medical electrical equipment safety, ensuring seamless integration across international markets. |
| 2. Are spare parts for the DEXIS Intraoral Scanner readily available, and what components are typically replaced? | DEXIS maintains an extensive global spare parts network with regional distribution hubs ensuring 48–72 hour delivery for critical components. Commonly replaced parts include scan tips (disposable and reusable variants), intraoral sensor caps, battery modules, and USB-C charging/data cables. Original Equipment Manufacturer (OEM) parts are serialized and tracked to maintain warranty compliance and performance integrity. Distributors are advised to stock high-turnover items under DEXIS’s 2026 Partner Inventory Program. |
| 3. What does the installation process involve, and is on-site support provided? | Installation of the DEXIS Intraoral Scanner includes hardware setup, software integration with DEXIS IQ or third-party CAD/CAM platforms (via open STL/DICOM export), and network configuration. Certified DEXIS Field Engineers provide on-site installation and calibration for new clinic deployments, including DICOM compliance verification and intra-clinic staff training. Remote pre-installation assessments are included in all 2026 purchase agreements to optimize workflow integration. |
| 4. What is the warranty coverage for the DEXIS Intraoral Scanner, and are extended service plans available? | The DEXIS Intraoral Scanner comes with a standard 3-year limited warranty covering defects in materials and workmanship, including sensor accuracy drift and internal electronic failure. Extended Service Agreements (ESA) are available for up to 5 years, including priority technical support, annual performance certification, and accidental damage protection (ADP) with one incident covered per year. Warranty is contingent upon registration within 30 days of delivery and use of OEM accessories. |
| 5. How does DEXIS ensure long-term serviceability and part availability beyond the warranty period? | DEXIS guarantees spare parts and technical support availability for a minimum of 7 years post-discontinuation of any scanner model, in compliance with ISO 13485 and FDA post-market requirements. The 2026 Service Lifecycle Commitment ensures firmware updates, cybersecurity patches, and compatibility with evolving digital dentistry standards (e.g., DICOM SR, AI-driven diagnostics). Distributors receive quarterly lifecycle notifications and end-of-support (EOS) alerts for proactive client upgrades. |
Note: Specifications and service terms are subject to change. Refer to the official DEXIS 2026 Product Datasheet and Service Agreement for contractual details.
Need a Quote for Dexis Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


