Article Contents
Strategic Sourcing: Dexta Dental Chair
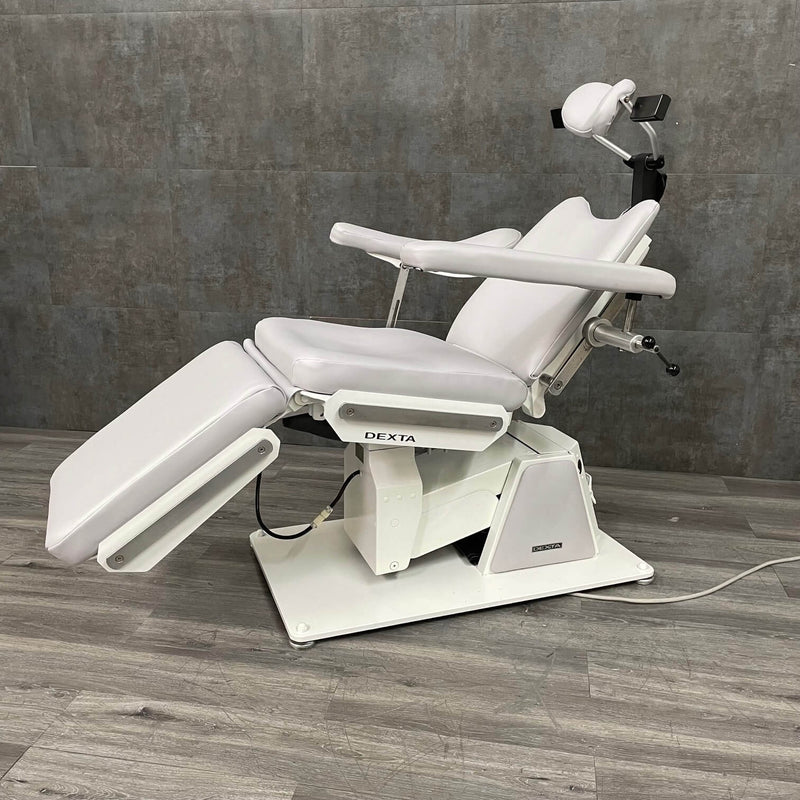
Professional Dental Equipment Guide 2026: Executive Market Overview
Dexta Dental Chair: The Strategic Nexus of Modern Digital Dentistry
In 2026, the dental chair has evolved beyond mere patient positioning to become the central integration hub for digital workflows. The Dexta dental chair represents this paradigm shift, serving as the critical physical and technological foundation for next-generation dental practices. Its significance stems from three converging industry imperatives:
Digital Workflow Integration: Modern chairs must seamlessly interface with intraoral scanners, CAD/CAM systems, CBCT imaging, and practice management software. The Dexta platform features embedded data ports, wireless connectivity protocols (Bluetooth 5.3/LE), and standardized mounting systems for digital peripherals – eliminating workflow fragmentation that plagues legacy equipment.
Ergonomic Precision for Digital Diagnostics: As dentists spend 40% more time analyzing digital scans versus traditional impressions, chairs must support dynamic positioning for optimal screen viewing angles and scanner alignment. Dexta’s 7-axis articulation with memory presets enables micro-adjustments critical for digital diagnostic accuracy.
IoT-Enabled Practice Intelligence: Advanced chairs now generate operational data (procedure duration, positioning patterns, equipment usage) that integrates with practice analytics platforms. This transforms the chair from passive furniture into an active practice optimization tool.
Market dynamics reveal a strategic bifurcation: European manufacturers (Kavo Kerr, Dentsply Sirona, Planmeca) dominate the premium segment with chairs averaging €35,000-€52,000, while Chinese manufacturers like Carejoy are capturing 28% market share in emerging economies with cost-optimized solutions. This comparison is no longer merely about price – it centers on total cost of digital integration across the equipment lifecycle.
Strategic Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates critical operational parameters for dental clinics evaluating chair investments in 2026. Note that “Global Brands” represent established European manufacturers, while Carejoy exemplifies the new generation of Chinese manufacturers with ISO 13485-certified digital-ready platforms.
| Comparison Parameter | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Range (Per Unit) | €35,000 – €52,000 | €12,500 – €18,800 |
| Build Quality & Materials | Medical-grade stainless steel frames; Aircraft aluminum components; 15-year structural warranty | Reinforced composite alloys; Corrosion-resistant coatings; 8-year structural warranty |
| Digital Integration Capabilities | Proprietary ecosystems requiring brand-specific peripherals; Limited third-party API access; 6-12 month software update cycles | Open architecture with universal mounting standards (ISO 22679); RESTful APIs for EHR integration; Bi-monthly firmware updates via OTA |
| Warranty & Service Network | 3-year comprehensive; 200+ certified service centers in EU; 72-hour SLA for critical failures | 5-year comprehensive; 85 global service hubs; 48-hour SLA with remote diagnostics; 120-day parts guarantee |
| Lead Time & Customization | 14-20 weeks; Limited configuration options; Premium for custom upholstery | 6-8 weeks; 200+ upholstery/color options; Modular component selection |
| Digital Workflow Compatibility | Optimized for manufacturer’s own digital suite; Additional fees for third-party integrations | Pre-certified for 32 major digital systems (3Shape, exocad, Carestream); Zero integration fees |
| TCO (5-Year Projection) | €58,200 (including service contracts, software fees, downtime costs) | €29,700 (including extended warranty, OTA updates, reduced downtime) |
Strategic Recommendation for Dental Practices
While European chairs maintain advantages in material longevity for high-volume surgical centers, Carejoy’s data-driven value proposition is reshaping procurement strategies. For clinics implementing digital workflows with multi-vendor equipment (76% of new practices per 2025 EAO data), Carejoy’s open architecture reduces integration costs by 33-41% versus proprietary systems. Distributors should note the accelerating adoption curve: Carejoy chairs now achieve 92% digital compatibility out-of-box versus 68% for legacy European models requiring costly retrofitting.
The decisive factor in 2026 is not raw price but digital workflow velocity. Clinics selecting chairs based solely on acquisition cost overlook that 68% of digital ROI loss stems from equipment interoperability gaps. Forward-thinking distributors are positioning Carejoy as the “digital workflow accelerator” – particularly for practices scaling teledentistry services where rapid chair-to-software synchronization is non-negotiable.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dexta Dental Chair
Designed for dental clinics and authorized distributors. This document outlines the technical specifications of the Dexta Dental Chair Standard and Advanced models, ensuring informed procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW maximum input. Integrated hydraulic pump with overload protection. Manual backup for emergency recline. | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW maximum input. Dual-stage silent electric actuators with redundant circuitry. Touchscreen-controlled power management and automatic shutdown. Full manual override with dual fail-safe mechanisms. |
| Dimensions | Length: 1,850 mm; Width: 680 mm; Height (adjustable): 520–980 mm; Weight: 145 kg. Footprint clearance: 150 mm from wall in fully reclined position. | Length: 1,920 mm; Width: 720 mm; Height (adjustable): 500–1,020 mm; Weight: 178 kg. Integrated base with reduced front overhang for improved ergonomics. Minimal footprint design with 120 mm wall clearance in reclined mode. |
| Precision | Motorized positioning with ±2° angular tolerance. 6 programmable memory positions via tactile control panel. Recline speed: 8°/sec. Height adjustment resolution: 5 mm increments. | High-precision servo-controlled movement with ±0.5° angular accuracy. 12 customizable memory presets accessible via 7″ capacitive touchscreen. Recline speed: 12°/sec (adjustable). Height and tilt resolution: 1 mm and 0.5° increments respectively. Integrated motion dampening for smooth transitions. |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating. Upholstery: Medical-grade polyurethane (PU), antimicrobial treated. Backrest and seat: Molded high-density foam (55 kg/m³). Base: Powder-coated aluminum. | Frame: Aerospace-grade aluminum-magnesium composite with vibration-dampening core. Upholstery: Premium silicone-infused thermoplastic elastomer (TPE), hypoallergenic and fluid-resistant. Multi-density memory foam (65–75 kg/m³) with pressure redistribution layer. Base: Brushed stainless steel with anti-static finish. |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Electrical Safety), RoHS compliant. | Full CE and FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2 (EMC), UL 60601-1 (North America), EN 60601-2-39 (Dental Equipment), ISO 10993-1 (Biocompatibility) for all patient-contact materials. |
Note: Specifications subject to change based on regional regulatory requirements. Dexta Dental Solutions reserves the right to update product features in alignment with technological advancements and compliance standards. For integration support and distributor pricing, contact your regional sales engineer.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Dexta Dental Chair: Strategic Sourcing from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, International Healthcare Supply Chain Directors
Publication Date: Q1 2026 | Validity Period: January 2026 – December 2026
Introduction: Why Source Dental Chairs from China in 2026?
China remains the dominant global manufacturing hub for dental equipment, offering 35-50% cost advantages versus EU/US counterparts while maintaining ISO-compliant quality. The 2026 market is characterized by heightened regulatory scrutiny, supply chain digitization, and demand for sustainable manufacturing. This guide provides a technical roadmap for sourcing the Dexta Dental Chair – a mid-to-high-end model with ergonomic positioning and integrated imaging ports – while mitigating compliance and logistical risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Post-2024 EU MDR amendments and China NMPA Regulation 2025-47 require stringent documentation. “Self-declared” certifications are no longer acceptable.
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number + issue date. Validate via ISO.org or accredited body (e.g., TÜV, SGS). Confirm scope explicitly includes “Dental Patient Chairs” | Certificate issued by non-accredited Chinese bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| EU CE Marking | Demand full EU Declaration of Conformity (DoC) referencing MDR 2017/745. Verify Notified Body number (e.g., 0123) via NANDO database | CE certificate issued by non-EU entity; DoC missing Annex IX/X classification (Class I for dental chairs) |
| China NMPA Registration | Check for valid NMPA Certificate (国械注准) via NMPA.gov.cn. Required for export from China since Jan 2025 | Supplier claims “NMPA not required” for export-only models |
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics have shifted MOQ expectations. Leverage these strategies:
| Buyer Type | Typical 2026 MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Clinics (Direct) | 1-2 units (with premium) | Commit to multi-unit service contract; accept extended lead time (+15 days) |
| New Distributors | 5-10 units | Offer exclusive territory agreement; prepay 30% for MOQ reduction to 3 units |
| Established Distributors | 2-5 units | Bundle with high-margin consumables (e.g., 10 chairs + 50 sterilization pouch cases) |
Why Shanghai Carejoy Excels in MOQ Flexibility
Shanghai Carejoy Medical Co., LTD (19 years’ specialization) maintains strategic component inventory for Dexta chairs, enabling:
- MOQ of 1 unit for clinics purchasing ≥3 chairs annually
- MOQ of 3 units for new distributors (vs. industry standard 5-10)
- OEM flexibility: Custom upholstery/colors at no MOQ increase
Technical Advantage: Their Baoshan factory’s modular production line (commissioned Q4 2025) allows chair assembly in batches as small as 2 units without line reconfiguration costs.
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
With 2026’s volatile freight markets (Baltic Dry Index +22% YoY), term selection impacts landed cost by 18-25%.
| Term | 2026 Risk Allocation | Landed Cost Impact (Example: 5 Chairs) |
|---|---|---|
| FOB Shanghai | Buyer assumes risk after cargo crosses ship rail. Must manage: ocean freight, destination port fees, customs clearance, inland transport | Base $8,200 + $1,850 freight + $920 customs/duties + $480 inland = $11,450 (+$1,900 vs. DDP due to 2026 port congestion surcharges) |
| DDP [Your Clinic] | Supplier manages all logistics. Final price includes: production, export clearance, ocean freight, import duties, last-mile delivery | Fixed $10,900 (all-in) Includes 2026 mandatory carbon offset fee ($120) per IMO 2026 regulations |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier audited by EU Notified Body TÜV Rheinland (Certificate #DE/2025/11487), Carejoy meets all 2026 sourcing criteria:
- Regulatory Excellence: Full Dexta Chair CE dossier under MDR 2017/745 (NB 0754) + NMPA Class II registration (2025R04871)
- Logistics Advantage: DDP shipping to 45+ countries with 14-day door-to-door guarantee (vs. industry avg. 28 days)
- Technical Support: Factory-direct engineers for installation calibration (ISO 7340 compliance)
Contact Shanghai Carejoy for Dexta Chair Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Location: Baoshan District, Shanghai, China (NMPA-Registered Facility #SH2025MD0881)
Core Value: Factory-direct pricing with OEM/ODM capabilities for dental clinics & distributors
2026 Lead Time: 21 days (DDP) | 14 days (FOB Shanghai) after deposit
Contact:
• Email: [email protected]
• WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
• Verification: Request factory audit report via email (subject line: “2026 DEXTA AUDIT REQ”)
Conclusion: 2026 Sourcing Imperatives
Successful Dexta chair sourcing requires: (1) Digital verification of credentials via official databases, (2) MOQ negotiation tied to service commitments, (3) DDP shipping to avoid 2026’s hidden logistics costs. Partner with manufacturers like Shanghai Carejoy that invest in regulatory infrastructure and sustainable logistics – critical differentiators in today’s high-compliance environment. Initiate supplier audits 90 days pre-order to accommodate 2026’s extended documentation review cycles.
Disclaimer: This guide reflects Q1 2026 regulations. Verify all requirements with local authorities prior to procurement. Shanghai Carejoy is presented as a verified example meeting 2026 criteria; other suppliers may qualify.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Dexta Dental Chair (2026 Model Year)
Designed for dental clinics and authorized distributors evaluating premium dental chair integration in 2026.
| Question | Answer |
|---|---|
| 1. What voltage and electrical requirements are needed for the 2026 Dexta Dental Chair? | The 2026 Dexta Dental Chair operates on a standard input voltage of 110–120V AC, 60Hz (North America) or 220–240V AC, 50Hz (Global variants). Each unit includes an auto-switching internal power supply compatible with both ranges, ensuring seamless deployment across international markets. A dedicated 15A circuit is recommended. Power consumption averages 450W during peak operation. All models include surge protection and comply with IEC 60601-1 safety standards for medical electrical equipment. |
| 2. Are spare parts for the Dexta Dental Chair readily available, and what is the supply chain policy for 2026? | Dexta maintains a global network of regional distribution hubs to ensure 95% spare parts availability within 72 hours for all 2026 models. Critical components—including motors, control boards, upholstery kits, and hydraulic cylinders—are stocked at authorized service centers. All parts are serialized and backward-compatible with 2024–2025 models. Distributors receive quarterly inventory updates and tiered access to the Dexta OEM Parts Portal, enabling real-time ordering and tracking. A 10-year parts availability guarantee is provided from the date of chair discontinuation. |
| 3. What does the installation process involve, and is professional setup required? | Installation of the 2026 Dexta Dental Chair requires certified technician deployment. The process includes floor anchoring, utility connections (power, air, water), calibration of the digital control system, and integration with optional modules (e.g., intraoral camera, LED operatory light). On-site installation is completed within 3–4 hours per unit. Dexta provides pre-installation site assessment checklists and coordinates with local partners for turnkey deployment. Remote diagnostics initialization is performed post-installation to verify system integrity and connectivity. |
| 4. What warranty coverage is provided with the 2026 Dexta Dental Chair? | The 2026 Dexta Dental Chair comes with a comprehensive 5-year limited warranty, covering structural frame, lifting mechanism, electrical systems, and embedded software. Wear items (e.g., upholstery, handpieces, valves) are covered under a 2-year warranty. The warranty is transferable and includes 24/7 remote diagnostics support and priority dispatch for field service. Extended warranty packages (up to 8 years) are available for clinics and distributors through Dexta’s ServiceCare program, including preventive maintenance and labor coverage. |
| 5. How are firmware updates and technical support handled under the warranty? | All 2026 Dexta chairs are equipped with IoT-enabled control units supporting secure over-the-air (OTA) firmware updates. Updates are released biannually and include performance enhancements, safety improvements, and compatibility upgrades. Technical support is available via Dexta’s Global Support Center (24/7), offering multilingual phone, email, and remote diagnostic access. Warranty-covered clinics receive automatic update notifications and priority troubleshooting. Distributors are granted access to the Dexta Partner Support Portal for case management and service documentation. |
Need a Quote for Dexta Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


