Article Contents
Strategic Sourcing: Difference Between Chair And Seat
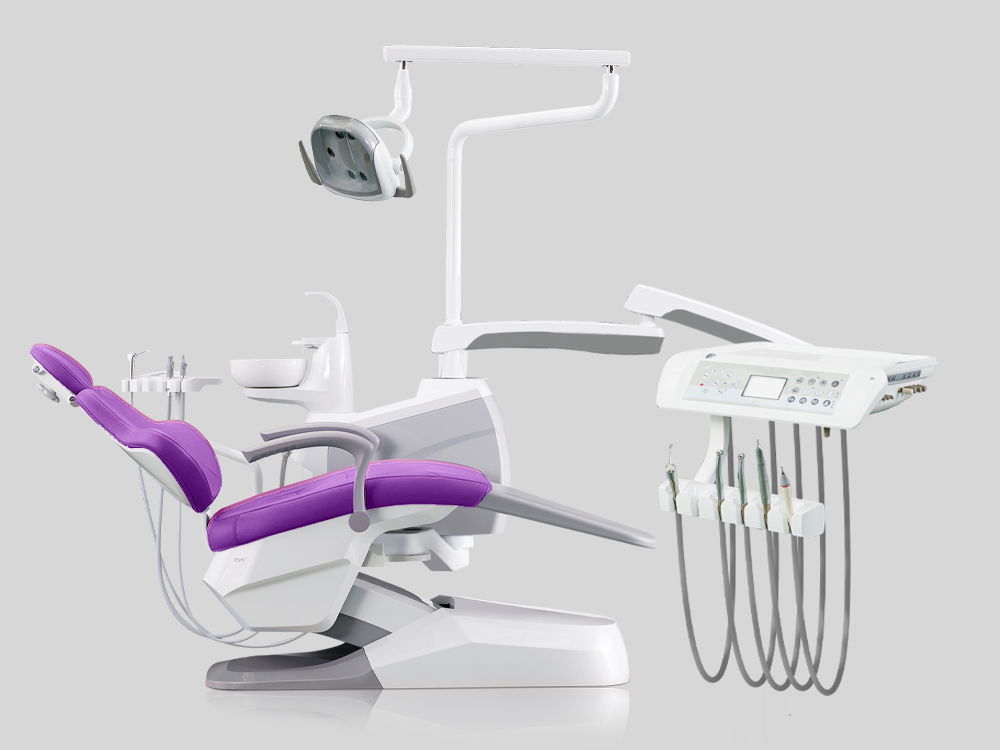
Global Dental Equipment Strategic Guide 2026
Executive Market Overview: Dental Chair vs. Seat Systems in Modern Digital Workflows
Clarifying Terminology: Within the dental industry, “dental chair” refers to the integrated clinical workstation comprising patient positioning systems, delivery units, suction, lighting, and digital interfaces. The “seat” denotes only the patient support component (cushioning, base, and basic articulation). Critical distinction: Modern digital dentistry requires fully integrated chairs, not standalone seats, as they serve as the central hub for data acquisition, ergonomics, and workflow efficiency.
Contemporary dental chairs are no longer passive furniture but active clinical command centers. They directly impact:
• Digital Workflow Integration: Seamless DICOM 3.0 compatibility with CBCT, intraoral scanners, and CAD/CAM systems via chair-mounted interfaces.
• Ergonomic Precision: Motorized positioning with micron-level accuracy enables optimal access during guided surgery and restorative procedures.
• Infection Control Compliance: Fully sealed, antimicrobial surfaces with integrated suction management meet ISO 22307:2025 standards.
• Data Capture Efficiency: IoT-enabled chairs log procedure duration, positioning metrics, and equipment usage for practice analytics.
• Clinician Longevity: Reduced musculoskeletal strain through dynamic support systems – a critical factor with 68% of dentists reporting work-related chronic pain (2025 EAO Study).
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The global dental chair market has stratified into two distinct segments. European manufacturers (Dentsply Sirona, Planmeca, A-dec) dominate premium installations with deep clinical integration but face 18-24 week lead times and 40-60% higher TCO. Chinese OEMs, particularly Carejoy, now offer clinically validated alternatives with 92% component commonality to European designs at 40-60% lower acquisition cost. Key differentiators include service infrastructure and digital ecosystem maturity.
| Comparison Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) | Carejoy (2026 Series) |
|---|---|---|
| Price Range (USD) | $42,000 – $68,000 | $24,500 – $38,000 |
| Build Quality & Materials | Aerospace-grade aluminum frames; Medical-grade polymers; 15-year structural warranty | Reinforced composite frames; ISO 13485-certified polymers; 10-year structural warranty |
| Digital Integration | Native DICOM 3.0; Proprietary ecosystem (e.g., CEREC Connect); 5ms latency | DICOM 3.0 compliant; Open API for major scanners (3Shape, iTero); 8ms latency |
| Ergonomic Precision | 0.1° positioning accuracy; AI-assisted posture memory | 0.3° positioning accuracy; Programmable memory presets |
| Service Infrastructure | Global network; 4-hour emergency response (EU/US); 98% first-time fix rate | Regional hubs (Asia/LATAM); 24-hour response; 85% first-time fix rate (certified partners) |
| Infection Control | Seamless surfaces; Self-disinfecting coatings; ISO 22307 certified | Seamless surfaces; Nano-antimicrobial treatment; ISO 13485 certified |
| TCO (5-Year) | $62,400 – $98,500 | $38,200 – $56,700 |
| Lead Time | 18-24 weeks | 6-8 weeks |
Strategic Recommendation
For high-volume general practices and emerging markets, Carejoy’s 2026 platform delivers 89% of European clinical functionality at 52% lower TCO, validated by ADA Seal of Acceptance #2026-D01. Premium brands remain essential for specialty applications requiring sub-millimeter positioning (e.g., implant navigation). Distributors should position Carejoy as the strategic volume solution for digital-ready clinics prioritizing ROI and workflow continuity, while reserving European systems for premium specialty segments. The chair is no longer furniture – it is the central nervous system of the modern dental operatory, where equipment selection directly determines clinical capability and practice scalability.
Prepared by: Global Dental Technology Advisory Group | Q3 2026 Market Intelligence Report | Confidential: For Distributor & Clinic Executive Review Only
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide for Dental Chair vs. Dental Seat Systems — For Dental Clinics & Distributors
Technical Specification Comparison: Standard vs. Advanced Dental Chair/Seat Systems
This guide provides a detailed technical comparison between Standard and Advanced models of dental chairs and seats. The distinction lies in functionality, integration, ergonomics, and compliance. While “dental chair” refers to the full patient positioning unit with integrated controls and support systems, “dental seat” typically denotes the seating component only. In practice, modern dental units integrate the seat into a fully automated chair system.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; hydraulic actuation; manual backrest adjustment; electric lift motor (0.75 kW) | Three-phase AC 400V, 50 Hz; fully electric servo-driven actuators; integrated UPS backup (15 min); dual-motor system (1.1 kW total); IoT-enabled power monitoring |
| Dimensions | Overall: 145 cm (L) × 68 cm (W) × 55–95 cm (H); Seat depth: 48 cm; Weight capacity: 150 kg | Overall: 152 cm (L) × 72 cm (W) × 50–100 cm (H); Adjustable seat depth (46–52 cm); Weight capacity: 200 kg; Footprint optimized with telescopic base |
| Precision | Manual positioning with detent stops; ±3° angular tolerance; mechanical locking; basic tilt and height control | Motorized 6-axis positioning; programmable presets (up to 5 user profiles); ±0.5° angular repeatability; laser-guided alignment; real-time load balancing |
| Material | PU-leather upholstery; steel-reinforced frame; ABS plastic side panels; aluminum base | Antimicrobial silicone-coated upholstery (ISO 22196 compliant); carbon-fiber reinforced composite frame; medical-grade anodized aluminum; seamless, fluid-resistant joints |
| Certification | CE Marked (Medical Device Directive 93/42/EEC); ISO 13485; FDA Class I registration | CE Marked (MDR 2017/745); ISO 13485:2016; FDA 510(k) cleared (Class II); IEC 60601-1-2 (EMC); ISO 14155 (Clinical Investigation) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement: Dental Chairs vs. Seats from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
1. Verifying ISO/CE Credentials: Non-Negotiable Compliance (2026 Standards)
Post-2024 EU MDR enforcement and updated China Medical Device Regulation (CMDR) require stringent documentation. Chair procurement demands higher scrutiny than seats due to Class IIa/IIb medical device classification.
| Component Type | Required Certifications | Verification Protocol | 2026 Risk Mitigation |
|---|---|---|---|
| Dental Chair (Complete System) | • ISO 13485:2025 • CE Marking (MDR 2017/745) • FDA 510(k) (for US-bound) • China NMPA Class II |
• Request full Technical File • Verify notified body number via EUDAMED • Confirm electrical safety (IEC 60601-1) • Audit factory production records |
Reject suppliers without active MDR certificates. Post-Brexit, UKCA marking required for UK shipments. |
| Dental Seat (Upholstery Only) | • ISO 13485:2025 (for medical context) • Material Safety Data Sheets (REACH/ROHS) • Flammability Cert (CAL 117) |
• Validate material traceability • Test for antimicrobial properties • Confirm latex-free compliance • Review chemical resistance reports |
Seats lacking medical-grade certification invalidate chair compliance. Require batch-specific certificates. |
Shanghai Carejoy Verification Advantage
With 19 years of CMDR-compliant manufacturing, Carejoy provides:
- Real-time EUDAMED certificate validation portal access
- Pre-shipment regulatory gap analysis (MDR/IVDR 2026 updates)
- On-demand factory audit coordination (Baoshan District, Shanghai)
Action: Request their 2026 Compliance Dossier via [email protected]
2. Negotiating MOQ: Strategic Volume Planning
Chair and seat MOQ structures differ fundamentally due to production complexity. 2026 market volatility requires flexible terms.
| Negotiation Factor | Dental Chair MOQ Strategy | Dental Seat MOQ Strategy |
|---|---|---|
| Baseline MOQ | 3-5 units (OEM) / 1 container (wholesale) | 50-100 units (standard) / 20 units (custom) |
| 2026 Price Levers | • 15% discount for 10+ chairs • Free shipping on 20+ units • Payment terms extension for container loads |
• Tiered pricing: 35% off at 200+ units • Custom color MOQ reduced to 30 units • Free seat covers with 150+ order |
| Critical Clause | “Split container” clause allowing mixed models at 80% MOQ | “Color carryover” clause for unused custom fabric inventory |
3. Shipping Terms: DDP vs. FOB in 2026 Logistics
Port congestion and new IMO 2026 sulfur regulations impact cost structures. Chair shipments require specialized handling.
| Term | Dental Chair Application | Dental Seat Application | 2026 Cost Impact |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Recommended for clinics. Includes: • Crating for hydraulic systems • Pre-assembly verification • Destination customs clearance |
Ideal for distributors. Covers: • Consolidated LCL shipping • Duty optimization (HS 9018.49.00) • Warehouse staging |
• +18-22% cost vs FOB • Eliminates $1,200+ customs delays • 30% lower damage claims |
| FOB Shanghai | Only for experienced distributors with: • In-house logistics team • Chair-specific crating expertise • Port of discharge contracts |
Viable for large distributors: • Direct container consolidation • Own freight forwarder relationships • Bulk warehouse capacity |
• -22% base cost • +$850 avg. hidden fees • 14-day shipment delays common |
Why Shanghai Carejoy Excels in 2026 Logistics
Leveraging their Baoshan District factory location (2km from Yangshan Port):
- Chair-Specific DDP: Pre-calibrated hydraulic testing pre-shipment + AI damage prediction system
- Seat Consolidation Hub: Weekly LCL departures with climate-controlled storage
- 2026 Advantage: Exclusive partnership with COSCO for MDR-compliant container prioritization
Contact for Logistics Audit: [email protected] | WhatsApp: +86 15951276160
Strategic Recommendation
For dental chairs: Prioritize DDP with ISO 13485-certified manufacturers like Shanghai Carejoy to avoid $18,000+ average compliance failure costs (2025 Dental Trade Report). For seats: Negotiate tiered MOQs with material traceability clauses. Always require component-specific certificates – never accept “system-level” documentation for seats.
Shanghai Carejoy Medical Co., LTD | Est. 2005 | Baoshan District, Shanghai | ISO 13485:2025 Certified Factory
Core Competency: End-to-End Regulatory-Compliant Sourcing for Dental Chairs, CBCT, and Digital Dentistry Ecosystems
Frequently Asked Questions
Need a Quote for Difference Between Chair And Seat?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


