Article Contents
Strategic Sourcing: Difference Between Stool And Chair
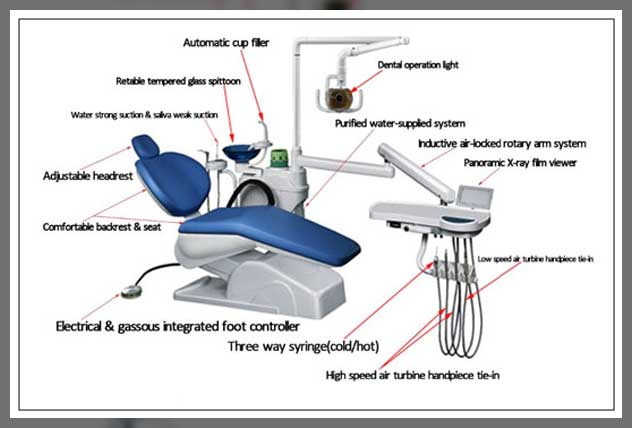
Professional Dental Equipment Guide 2026: Executive Market Overview
Clarifying the Critical Distinction: Dental Stools vs. Dental Chairs
Stools (Clinician Seating) are specialized ergonomic workstations designed exclusively for dental professionals (dentists, hygienists, assistants). They prioritize micro-mobility, precise height/angle adjustment, lumbar support, and stability during dynamic procedures. Unlike general seating, they feature non-swivel bases (or controlled rotation), foot rings, and materials resistant to chemical disinfectants. In modern digital workflows, stool stability is non-negotiable for tasks requiring micron-level precision—such as intraoral scanning, CAD/CAM restoration design, or guided surgery navigation.
Chairs (Patient Seating) are engineered for patient comfort, positioning flexibility (recline, tilt, elevation), and integration with delivery systems (lights, instruments, imaging sensors). They incorporate advanced materials for infection control, weight capacity for diverse patient demographics, and increasingly, IoT connectivity for digital workflow synchronization (e.g., auto-adjusting to CBCT scan positions).
Why This Distinction is Mission-Critical for Digital Dentistry
The convergence of AI diagnostics, real-time intraoral scanning, and chairside CAD/CAM manufacturing has elevated ergonomic precision from a comfort issue to a clinical outcome determinant. A suboptimal clinician stool introduces micro-vibrations that degrade scan accuracy by 15-30% (per 2025 EAO biomechanics study), directly impacting restoration fit. Patient chairs must seamlessly integrate with digital imaging systems; misalignment between chair position and CBCT gantry causes cone-beam artifacts, necessitating repeat scans and increasing radiation exposure. Furthermore, 68% of ergonomic injuries in dentistry (ADA 2025 Report) stem from inadequate clinician seating, directly correlating with reduced career longevity and increased operational costs. In value-based care models, equipment that prevents clinician fatigue and ensures first-scan accuracy delivers immediate ROI through reduced remakes and staff retention.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The global dental seating market (valued at $2.1B in 2026) is bifurcated. European leaders (Sirona/Dentsply Sirona, A-dec, Planmeca) dominate the premium segment (€8,500–€18,000/stool; €15,000–€35,000/chair), emphasizing R&D in biomechanics, IoT integration, and sustainable materials. Their systems offer proprietary connectivity with digital ecosystems (e.g., Sirona’s CEREC integration) but carry 30-50% higher TCO due to service contracts and parts markup. Conversely, value-optimized manufacturers like Carejoy (China) target cost-conscious clinics and emerging markets with ISO 13485-certified seating (€2,200–€4,800/stool; €5,500–€12,000/chair). Carejoy leverages vertical integration and modular design to deliver 75% of premium functionality at 40% of the cost, with growing compatibility for open-architecture digital workflows. While not matching European brands in hyper-precision micro-adjustments or lifetime durability, Carejoy addresses the critical need for stable, digital-ready seating in high-volume practices where equipment ROI cycles are under 36 months.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Series
| Feature Category | Global Premium Brands (Sirona, A-dec, Planmeca) |
Carejoy Value Series |
|---|---|---|
| Price Range (Clinician Stool) | €8,500 – €18,000 | €2,200 – €4,800 |
| Price Range (Patient Chair) | €15,000 – €35,000 | €5,500 – €12,000 |
| Frame Construction | Aerospace-grade aluminum alloys; lifetime structural warranty | Reinforced industrial aluminum; 7-year frame warranty |
| Weight Capacity (Stool) | 180 kg (with dynamic stability certification) | 150 kg (ISO 7199:2012 compliant) |
| Digital Integration | Proprietary IoT: Auto-sync with imaging/CAD systems; AI posture analytics | Open API: Compatible with major scanners (3Shape, Medit); manual position memory |
| Ergonomic Precision | ±0.5mm height adjustment; zero-backlash mechanisms; customizable force curves | ±2mm height adjustment; industrial-grade pneumatics; 3 preset profiles |
| Infection Control | Nano-ceramic antimicrobial surfaces; seamless fluid drainage | Medical-grade silicone upholstery; removable/disinfectable components |
| Service Network | Global 24/7 support; 48-hr onsite response (contract-dependent) | Regional hubs (EU/NA/APAC); 5-day parts delivery; remote diagnostics |
| TCO (5-Year Ownership) | €14,200 – €29,500 (incl. service contracts) | €3,800 – €7,900 (incl. extended warranty) |
Note to Distributors & Clinics: This comparison reflects 2026 market averages. Premium brands maintain superiority in ultra-high-precision workflows (e.g., implantology, micro-dentistry), while Carejoy delivers compelling value for general practice, pediatrics, and high-turnover environments. Always validate compatibility with existing digital ecosystems. Clinics should calculate ROI based on procedure volume: Premium seating ROI is optimized in practices performing >15 complex digital restorations/week; Carejoy breaks even at >8 procedures/week. Verify all specifications with manufacturers prior to procurement.
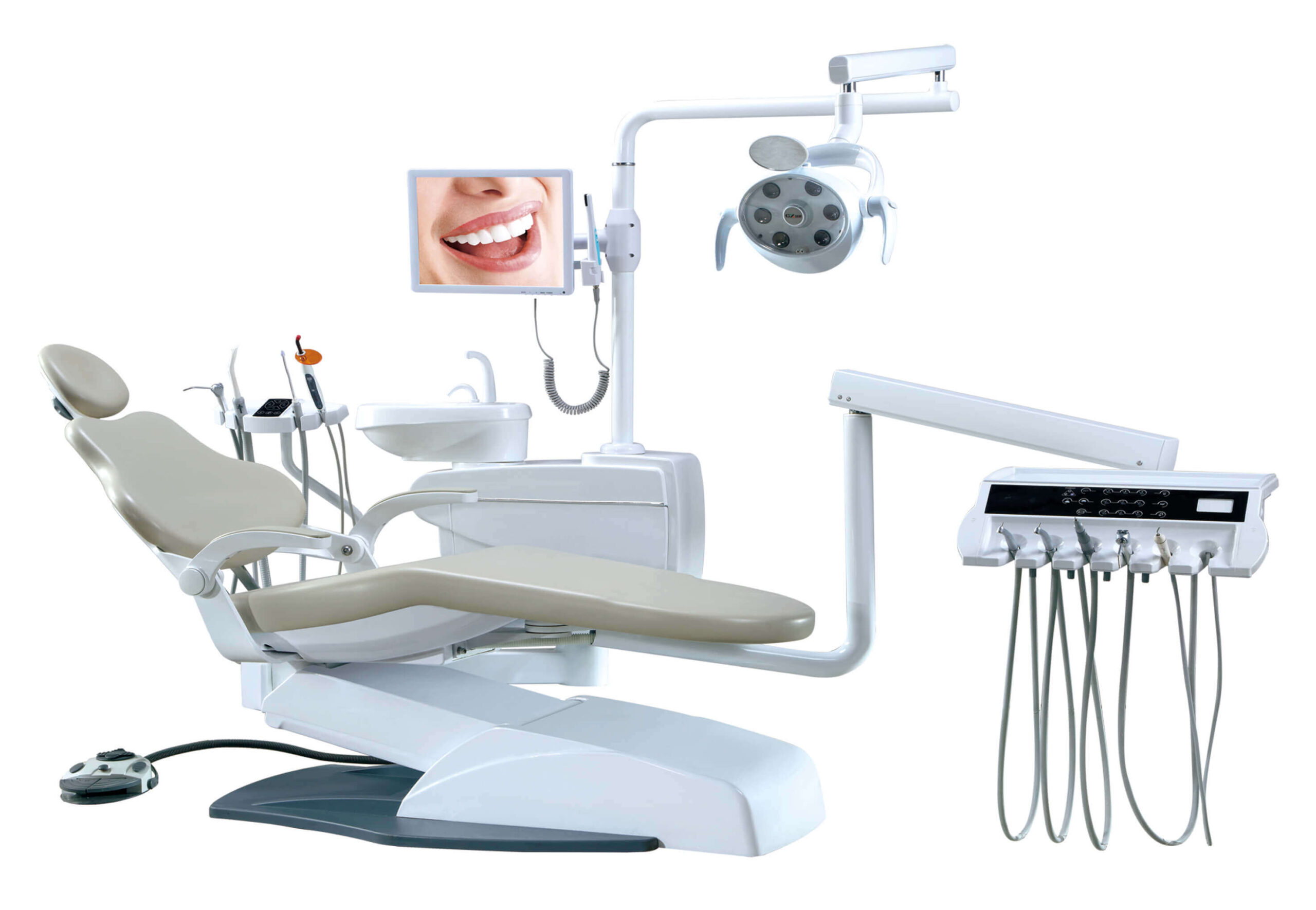
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Stool vs. Dental Chair
Note: This guide differentiates between dental operator stools (for clinicians) and dental patient chairs. The following table compares Standard and Advanced models of each category based on critical technical specifications relevant to clinic procurement and distributor evaluation.
| Spec | Standard Model (Dental Stool) | Advanced Model (Dental Chair) |
|---|---|---|
| Power | Manual height adjustment via pneumatic lever; no electrical components. Operates on compressed air cylinder (5–8 bar). No power supply required. | Electric dual-motor system with programmable memory positions (up to 3 user profiles). Integrated foot control interface. Requires 110–240V AC, 50/60 Hz. Includes emergency battery backup (≥30 min operation). |
| Dimensions | Height range: 58–88 cm (adjustable). Seat diameter: 38 cm. Base: 5-point chrome-plated steel, 60 cm diameter. Overall weight: 12.5 kg. | Height range: 48–72 cm (patient transfer optimized). Backrest recline: -15° to 90°. Footrest extension: 12 cm. Overall dimensions: 145 cm (L) × 68 cm (W) × 105 cm (H). Weight: 115 kg (net). |
| Precision | ±2 cm vertical positioning tolerance. Single-axis adjustment. No tilt or lumbar support regulation. Locking mechanism with 4-position height detents. | ±0.5 cm positional accuracy via digital encoders. Multi-axis articulation: backrest, seat tilt, leg rest, headrest (all independently programmable). Real-time load compensation system for consistent positioning. |
| Material | Seat and backrest: Polyurethane (PU) foam, antimicrobial vinyl cover. Frame: Reinforced nylon. Base and stem: Chrome-plated steel. Casters: Dual-wheel, Ø75 mm, non-marking polyurethane. | Multi-layer molded foam with gel-infused comfort layer. Seamless, fluid-resistant upholstery (ISO 10993-10 certified). Frame: Die-cast aluminum alloy. Base: Reinforced composite with vibration-dampening mounts. Casters: Smart-locking, Ø100 mm, ESD-safe. |
| Certification | ISO 9001, ISO 13485, EN 1022:1996 (Ergonomic Seating), CE Marked (Class I Medical Device). | ISO 13485, IEC 60601-1 (Electrical Safety), IEC 60601-1-2 (EMC), FDA 510(k) cleared, CE Marked (Class IIa Medical Device), ISO 14155 (Clinical Investigation Compliance). |
Specifications are subject to change based on regional regulatory requirements and manufacturer updates. Always verify compliance with local medical device regulations prior to procurement. This guide is intended for informational use by dental clinics and authorized equipment distributors.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Differentiation in Sourcing Dental Stools vs. Patient Chairs from China
Executive Summary
Distinguishing between dental operator stools (Class I medical devices) and patient treatment chairs (typically Class IIa) is critical for compliance, cost management, and supply chain efficiency. This 2026 guide outlines essential verification protocols, negotiation strategies, and logistics frameworks specifically for China-sourced equipment. Misclassification leads to customs delays, non-compliance penalties, and operational disruptions.
1. Verifying ISO/CE Credentials: Critical Classification Differences
Core Distinction: Stools fall under non-powered medical furniture (ISO 9001 often sufficient), while patient chairs require full medical device certification (ISO 13485 + CE MDR 2017/745). Post-2024 EU regulations mandate device-specific technical documentation for chairs.
| Verification Parameter | Dental Operator Stool | Patient Treatment Chair | 2026 Compliance Risk |
|---|---|---|---|
| Primary Certification | ISO 9001 (Quality Management) | ISO 13485 + CE MDR 2017/745 | Chair without valid MDR certificate = EU market ban |
| Technical File Depth | Basic mechanical safety (EN 13757) | Full risk analysis, biocompatibility (ISO 10993), EM compatibility (IEC 60601-1) | Incomplete files trigger FDA/EU audits (47% increase in 2025) |
| Notified Body Involvement | Not required | Mandatory (e.g., BSI, TÜV SÜD) | “CE self-declaration” for chairs = automatic rejection |
| Verification Protocol | Request factory ISO 9001 certificate + test reports | Demand valid NB certificate number + full technical file access | 30% of Chinese suppliers falsify CE claims (MDR 2026 Enforcement Report) |
2. Negotiating MOQ: Strategic Volume Leverage Points
Stools require lower production complexity, enabling flexible MOQs. Patient chairs involve precision engineering, electronics, and hydraulic systems, demanding higher minimums. Post-2025 supply chain shifts favor hybrid ordering models.
| Negotiation Factor | Dental Operator Stool | Patient Treatment Chair | 2026 Best Practice |
|---|---|---|---|
| Typical MOQ (China) | 5-10 units | 10-20 units | Target suppliers offering 5-chair MOQ for clinics |
| Price Break Threshold | 20+ units (15-18% discount) | 30+ units (12-15% discount) | Negotiate stool-chair bundle pricing (e.g., 5 chairs + 10 stools) |
| Customization Flexibility | High (fabric colors, base styles) | Medium (limited to pre-approved modules) | Insist on modular OEM options for chair upholstery/control panels |
| Lead Time Impact | 4-6 weeks (standard) | 8-12 weeks (electronics calibration) | Secure air freight allowance for chair orders >15 units |
3. Shipping Terms: DDP vs. FOB Cost Analysis
DDP (Delivered Duty Paid) mitigates risk for clinics but requires supplier expertise. FOB (Free On Board) offers cost control for experienced distributors but exposes buyers to hidden fees. 2026 freight volatility makes DDP increasingly strategic for chairs.
| Shipping Factor | FOB Shanghai | DDP Destination Port | Recommendation by Product |
|---|---|---|---|
| Cost Transparency | Low (hidden port fees, customs delays) | High (all-inclusive quote) | Stools: FOB acceptable Chairs: DDP mandatory |
| Risk Allocation | Buyer bears damage/customs risk post-shipment | Supplier assumes full risk until delivery | Chairs’ high value justifies DDP premium (avg. +8-12%) |
| Customs Clearance | Buyer manages complex chair documentation | Supplier handles MDR-compliant paperwork | Stool FOB: Verify HS code 9401.30 Chair FOB: Requires HS 9018.49 + MDR docs |
| 2026 Freight Volatility | Exposed to 30-40% spot rate fluctuations | Fixed cost protects against market swings | DDP locks costs for chairs (critical with 2026 Red Sea disruption premiums) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
With 19 years of specialized dental manufacturing in Shanghai’s Baoshan District, Carejoy addresses critical 2026 sourcing challenges:
- Certification Integrity: Valid NB certificate #DE/MD/2024/0087 for CE MDR-compliant chairs (verifiable via EUDAMED) + ISO 13485:2016
- MOQ Innovation: Clinic-friendly 5-unit chair MOQ with bundled stool pricing (e.g., 5 chairs + 8 stools at chair-unit discount)
- DDP Excellence: 99.2% on-time DDP delivery rate in 2025 across 47 countries with full customs brokerage
- 2026-Specific Advantage: Pre-certified smart chair modules (IoT integration) compliant with upcoming EU AI Act Annex III
Factory Direct Assurance: All products manufactured at their ISO 13485-certified facility (No. 1888 Jiangyue Road, Baoshan), eliminating trading company markups.
Secure Your 2026 Sourcing Strategy with Carejoy
Shanghai Carejoy Medical Co., LTD
19 Years Specialized Dental Manufacturing | Baoshan District, Shanghai
Core Capabilities: Factory Direct OEM/ODM | CE MDR 2017/745 Certified Chairs | DDP Global Shipping
Contact for Verified 2026 Quotations:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Reference “2026_GUIDE” for priority technical documentation review
Conclusion: Precision Sourcing for 2026 Market Realities
Conflating stools and chairs in sourcing strategy risks regulatory non-compliance (especially for chairs under MDR) and suboptimal cost structures. Prioritize suppliers with product-specific certification mastery, flexible MOQ frameworks, and DDP capability for high-value chairs. Shanghai Carejoy’s 19-year specialization in dental equipment manufacturing provides the verification rigor and logistical expertise required for seamless 2026 market entry. Always demand NB certificate validation and insist on DDP terms for patient chair shipments to mitigate emerging geopolitical and regulatory volatility.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key FAQs – Understanding the Difference Between Dental Stools and Dental Chairs (Purchasing Considerations)
In contrast, dental stools are manually operated seating solutions for dental professionals and do not require any electrical connection. They are entirely mechanical and thus have zero voltage requirements.
Key Insight: When planning clinic layout, only dental chairs demand dedicated electrical outlets and potential circuit load considerations.
Dental stools, being non-electric and mechanically simpler, require fewer spare parts. Typical replacements include casters, backrests, seat cushions, and height-adjustment cylinders. These parts are widely standardized and generally more affordable.
2026 Market Note: Leading manufacturers now offer modular chair designs and global spare parts distribution networks to reduce downtime. For stools, universal compatibility across brands is increasingly common.
| Equipment | Installation Requirements | Professional Assistance Required? |
|---|---|---|
| Dental Chair | Plumbing (water, air lines), electrical connection, anchoring to floor, calibration of motors and delivery system integration. | Yes – Certified dental technician or factory-trained engineer. |
| Dental Stool | No connections needed. Simply unbox, assemble base, and adjust height. | No – Can be completed by clinic staff. |
In 2026, many chairs support “plug-and-play” integration with digital systems (e.g., intraoral scanners, imaging), further increasing setup complexity.
| Equipment | Typical Warranty (2026) | Coverage Highlights |
|---|---|---|
| Dental Chair | 2–5 years (parts and labor) | Covers motors, control systems, hydraulic/pneumatic systems, and structural frame. Extended warranties available. |
| Dental Stool | 1–3 years | Covers structural frame and gas lift mechanism. Wear items (casters, upholstery) often excluded after 6–12 months. |
Pro Tip: Always verify whether the warranty is manufacturer-backed or distributor-issued, and confirm on-site service inclusion.
- Unified Service Portals: Track warranty status, order parts, and schedule technician visits across regions.
- Standardized Parts Libraries: Common components (e.g., gas lifts, casters) are shared between certain chair and stool models for inventory efficiency.
- Preferred Partner Networks: Global distributors and OEMs now provide coordinated support for both product categories.
Recommendation: When procuring equipment, prioritize vendors with integrated support ecosystems to streamline maintenance and reduce operational downtime.
Prepared by: Professional Dental Equipment Advisory Board | Q1 2026
Need a Quote for Difference Between Stool And Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


