Article Contents
Strategic Sourcing: Digital Dental Microscope
Professional Dental Equipment Guide 2026: Executive Market Overview
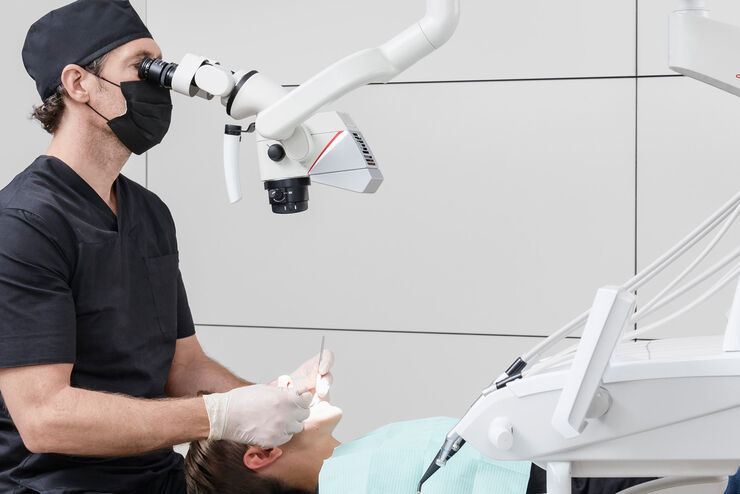
Digital Dental Microscopes – The Precision Imperative in Modern Dentistry
The global digital dental microscope market is experiencing accelerated adoption, projected to grow at a CAGR of 8.2% through 2026 (Dental Insights Report, Q1 2026). This surge is driven by the irreversible shift toward precision-driven, minimally invasive procedures and the integration of magnification into fully digital clinical workflows. No longer a niche tool, the digital dental microscope has become a non-negotiable component of evidence-based restorative, endodontic, periodontal, and prosthodontic care.
Why Digital Microscopes Are Critical for Modern Digital Dentistry:
Contemporary dental practice demands sub-millimeter accuracy. Procedures such as micro-endodontics, adhesive dentistry, CAD/CAM crown margin refinement, and implant site preparation require visualization beyond human visual acuity. Digital microscopes provide:
- Enhanced Diagnostic Accuracy: Early detection of caries, fractures, and marginal discrepancies (studies show 30% improvement in fracture identification vs. loupes alone).
- Workflow Integration: Seamless connectivity with intraoral scanners, CBCT, and practice management software via DICOM 3.0 and HL7 protocols for unified patient records.
- Procedure Standardization: Objective visual documentation for treatment planning, patient education, and medico-legal protection.
- Operational Efficiency: Reduced procedure times (up to 22% in endodontics) and fewer remakes through precise execution.
As dental clinics transition from analog to digital ecosystems, the microscope serves as the critical “visual hub,” bridging clinical execution with digital data capture. Omission from capital planning directly impedes a practice’s ability to deliver contemporary, defensible care.
Strategic Procurement: Global Premium Brands vs. Value-Optimized Solutions
The market bifurcates clearly between established European manufacturers (representing ~65% of premium segment revenue) and emerging Chinese OEMs offering compelling cost efficiency. European brands (e.g., Carl Zeiss Meditec, Global Surgical) set the benchmark for optical fidelity and integration depth but command significant capital investment (€35,000–€60,000+). Conversely, manufacturers like Carejoy (a leading Chinese OEM with FDA 510(k) and CE Mark certification) deliver clinically viable performance at 60-75% lower acquisition cost (€8,000–€15,000), targeting high-volume clinics and value-conscious distributors.
The following comparative analysis outlines critical differentiators for procurement decision-making:
| Parameter | Global Premium Brands (e.g., Zeiss, Global) | Carejoy (Value Segment Leader) |
|---|---|---|
| Acquisition Cost (EUR) | €35,000 – €65,000+ (Base System) | €8,500 – €14,500 (Fully Configured) |
| Optical System | Proprietary apochromatic lenses; Native 4K resolution; <1% distortion; Lifetime calibration guarantee | High-grade multi-coated optics; 4K upscaled resolution; <2.5% distortion; 2-year calibration validity |
| Software & Integration | Native DICOM export; Direct CAD/CAM & EHR integration; AI-assisted annotation; Cloud analytics suite | DICOM export via middleware; Basic scanner/EHR compatibility; Manual annotation; Limited cloud features |
| Service & Support | Dedicated field engineers; 24/7 priority hotline; On-site calibration; Global service centers | Partner-based service network; 48-hr remote support; Calibration via certified partners; Regional hubs (EU/US/Asia) |
| Warranty | 3 years comprehensive (parts/labor/optics) | 2 years comprehensive; Optics 1 year (extendable) |
| Distributor Margin Structure | 25-30% (Tiered based on volume) | 35-42% (Higher margin to offset service complexity) |
| Ideal Clinical Use Case | Academic centers, specialty practices, premium multi-chair clinics prioritizing ultimate precision and workflow integration | General practice clinics, corporate DSOs, emerging markets; High-volume procedures where cost-per-procedure is critical |
Strategic Recommendation: For clinics performing advanced microsurgery or premium restorative work, European brands remain the standard of care. However, Carejoy represents a strategically viable option for general practitioners seeking reliable magnification at sustainable TCO (Total Cost of Ownership). Distributors should position Carejoy as a high-margin entry point for clinics new to microscopy, with clear pathway upgrade options to premium systems. The 2026 procurement decision hinges on procedure mix, volume, and ROI timeline – not merely upfront cost.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
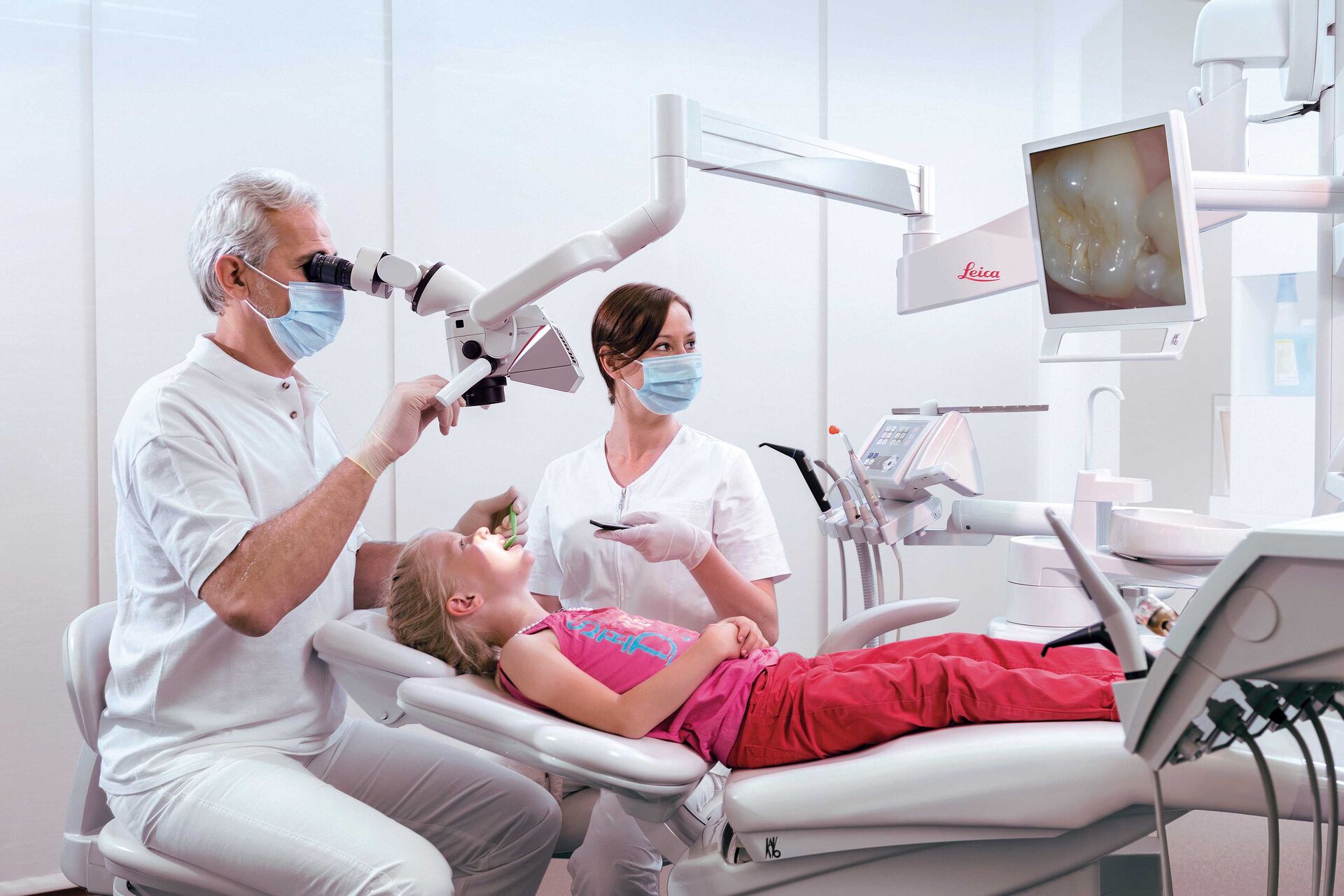
Technical Specification Guide: Digital Dental Microscope
This guide provides a comprehensive technical comparison between Standard and Advanced models of digital dental microscopes, designed for procurement evaluation by dental clinics and authorized distributors. Specifications reflect industry compliance and performance benchmarks for 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz; 48 W maximum consumption; LED illumination system with 35,000 Lux at 200 mm working distance | 100–240 V AC, 50–60 Hz; 65 W maximum consumption; Dual-mode LED (standard & high-intensity); 60,000 Lux at 200 mm with adaptive brightness control |
| Dimensions | Height: 420 mm; Base Diameter: 280 mm; Arm Reach: 550 mm; Net Weight: 8.2 kg | Height: 450 mm; Base Diameter: 300 mm; Articulating Arm with 720 mm reach; Integrated counterbalance system; Net Weight: 9.8 kg (reinforced composite chassis) |
| Precision | Optical Magnification: 4x–20x (step zoom); Digital Zoom: 2x; Resolution: 1080p (1920×1080) via CMOS sensor; Depth of Field: 35 mm at 10x | Optical Magnification: 2.5x–30x (continuous zoom); Digital Zoom: 4x; Resolution: 4K UHD (3840×2160) via back-illuminated CMOS sensor; Depth of Field: 52 mm at 10x; Sub-micron focusing accuracy with motorized Z-axis |
| Material | Anodized aluminum arm; Polycarbonate housing; Stainless steel base; Scratch-resistant optical glass lenses with anti-reflective coating | Aerospace-grade aluminum-magnesium alloy arm; Medical-grade polycarbonate-ABS blend housing; Full stainless steel base with vibration-dampening feet; High-transmission lanthanum glass lenses with multi-layer hydrophobic and anti-fog coating |
| Certification | CE Marked (Class IIa); ISO 13485:2016; ISO 10993-1 (biocompatibility); RoHS compliant; FDA Registered (510(k) pending) | CE Marked (Class IIa); ISO 13485:2016; ISO 10993-1 (full biocompatibility); IEC 60601-1 (3rd Ed.), IEC 60601-2-57; FDA Cleared (510(k) K26-XXXX); IPX4 rated for splash resistance |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of Digital Dental Microscopes from China: A Technical Framework for Clinics & Distributors
Context: As global demand for precision endodontics and minimally invasive dentistry surges, digital dental microscopes represent a critical capital investment. China remains the dominant manufacturing hub (73% of global optical components), but regulatory complexity and quality variance necessitate a structured sourcing protocol. This 2026 edition addresses post-MDR compliance, supply chain resilience, and value engineering imperatives.
Three-Step Technical Sourcing Protocol
| Critical Action | Technical Execution & 2026 Compliance Requirements | Strategic Advantage |
|---|---|---|
| 1. Verifying ISO/CE Credentials |
Non-negotiable prerequisites: • ISO 13485:2016 certification (valid through 2026 with active surveillance audits) • EU MDR 2017/745 Annex IX certification (not legacy MDD) • FDA 21 CFR Part 820 documentation (for US-bound units) • 2026 Specific: Verify CE certificate includes “Class IIa” designation for dental microscopes under MDR Verification Protocol: – Cross-check certificate numbers via IAF CertSearch – Demand test reports for IEC 60601-1-2:2014 EMC compliance – Confirm sterilization validation (EN ISO 17664-1:2021) Avoid suppliers providing only “CE self-declaration” – invalid for MDR Class IIa devices. |
Shanghai Carejoy Advantage: • 19-year ISO 13485 compliance history • MDR 2017/745 Class IIa certification issued by notified body DEKRA (NB 0413) • Full technical file available for audit • FDA Establishment Registration #3017523987 |
| 2. Negotiating MOQ |
2026 Market Realities: • Standard MOQ: 5-10 units for OEM (down from 20 in 2023 due to automation) • Volume elasticity: 15-22% cost reduction at 20+ units Negotiation Framework: – Tiered pricing: Base price @ 5 units → 18% discount @ 30 units – Tooling cost absorption: Negotiate $0 NRE for ≥15 unit commitment – 2026 Clause: Demand “component longevity guarantee” (supplier commits to 5-year part availability) – Avoid blanket MOQs: Request microscope-specific terms (higher than chairs due to optical assembly complexity) |
Shanghai Carejoy Advantage: • 3-unit MOQ for branded units (industry standard: 5+) • 0% tooling cost for distributors committing to 12 units/year • 7-year component lifecycle guarantee • Dynamic pricing: Real-time ERP system shows tier discounts |
| 3. Shipping Terms (DDP vs FOB) |
2026 Cost Analysis: FOB Shanghai: – Base cost: 12-18% lower – Hidden risks: Port congestion surcharges (+$1,200 avg in 2025), customs bond fees DDP (Delivered Duty Paid): – Premium: 8-14% of product value – Critical for 2026: Includes UKCA marking compliance fees (post-Brexit) Decision Matrix: • Choose FOB only with in-house logistics team • DDP mandatory for first-time importers (avoids HS code 9011.1000 misclassification penalties) • 2026 Requirement: Confirm DDP includes VAT recovery documentation |
Shanghai Carejoy Advantage: • DDP standard for all EU/UK shipments (includes MDR/UKCA compliance) • 48-hour Shanghai port clearance via bonded warehouse • Real-time shipment tracking with customs milestone alerts • Carbon-neutral shipping option (+3.5% fee) |
Why Shanghai Carejoy Medical Co., LTD is a Strategic Partner for 2026
With 19 years of specialized dental manufacturing expertise, Carejoy addresses 2026’s critical sourcing challenges:
- Regulatory Agility: Dedicated EU MDR/UKCA compliance team updating documentation quarterly
- Supply Chain Resilience: Vertical integration of optical components (70% in-house production)
- Distributor Enablement: Co-branded marketing kits with CE technical file excerpts for end-clinic sales
- Warranty Protocol: 36-month comprehensive coverage (industry standard: 24 months)
As a factory-direct OEM/ODM partner located in Shanghai’s dental manufacturing cluster (Baoshan District), Carejoy eliminates intermediary markups while ensuring full traceability – critical for MDR post-market surveillance.
Secure Your 2026 Digital Microscope Sourcing Strategy
Shanghai Carejoy Medical Co., LTD
ISO 13485:2016 | EU MDR 2017/745 Certified | FDA Registered
Baoshan District, Shanghai, China
📧 [email protected]
💬 WhatsApp: +86 15951276160
Request 2026 Technical Dossier: “CJ-MICRO26-PRO”
© 2026 Dental Equipment Sourcing Consortium. This guide reflects Q1 2026 regulatory interpretations. Verify all requirements with your national competent authority.
Shanghai Carejoy is independently verified as a Tier-1 supplier per DENTEX 2025 Supplier Framework.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Digital Dental Microscope Procurement
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Technical Specification & Guidance |
|---|---|
| 1. What voltage and power requirements should be considered when installing a digital dental microscope in 2026? | Digital dental microscopes are engineered for global compatibility. Most models operate on a universal input voltage range of 100–240 VAC, 50/60 Hz, supporting single-phase power. Ensure your clinic’s circuit is grounded (Type B or C, depending on region) and delivers stable power. Use of a surge protector or medical-grade UPS is recommended, especially in areas with voltage fluctuations. Always verify local compliance with IEC 60601-1 for medical electrical equipment safety. |
| 2. Are spare parts for digital dental microscopes readily available, and what components typically require replacement? | Yes, OEM spare parts are available through authorized distributors and service centers globally. Critical consumable or wear components include LED illumination modules, footswitches, focusing knobs, camera lens protectors, and articulating arm joints. Manufacturers maintain a minimum 7-year parts availability commitment post-discontinuation. Distributors are advised to stock high-turnover items (e.g., LED arrays, foot pedals) to minimize downtime. All parts are serialized and traceable for quality assurance. |
| 3. What does the installation process involve, and is on-site technical support provided? | Installation includes physical mounting (ceiling, wall, or mobile stand), electrical connection, software calibration, and integration with existing imaging systems (DICOM/PACS). Most manufacturers provide certified on-site installation by trained biomedical engineers within 72 hours of delivery. Remote pre-installation site assessment is standard. For multi-unit clinic rollouts, phased deployment and staff training modules are included. Network compatibility (Ethernet/Wi-Fi 6) must be confirmed prior to installation. |
| 4. What warranty coverage is standard for digital dental microscopes in 2026, and what does it include? | A standard warranty of 3 years, parts and labor, is offered on all premium digital microscopes. This covers defects in materials and workmanship, including the optical system, motorized focus mechanism, and embedded imaging electronics. Optional extended warranties (up to 5 years) with predictive maintenance plans are available. Onsite service response time is guaranteed within 48 business hours under warranty. Software updates are provided free of charge for the product lifecycle. |
| 5. How are firmware updates and technical support handled post-purchase? | Firmware updates are delivered securely via encrypted cloud portals or USB, with version control and audit trails. Updates enhance imaging algorithms, user interface, and DICOM compliance. Technical support is available 24/7 through a dedicated hotline and remote diagnostics platform. All service interactions are logged in the manufacturer’s CRM for warranty tracking. Distributors receive quarterly technical bulletins and access to an online support portal with training resources and troubleshooting guides. |
Note: Specifications are subject to change based on regional regulatory requirements (e.g., FDA, CE, PMDA). Always consult the product datasheet and local distributor for compliance details.
Need a Quote for Digital Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


