Article Contents
Strategic Sourcing: Digital Dental X Ray Equipment
Professional Dental Equipment Guide 2026
Executive Market Overview: Digital Dental X-Ray Equipment
Criticality in Modern Digital Dentistry
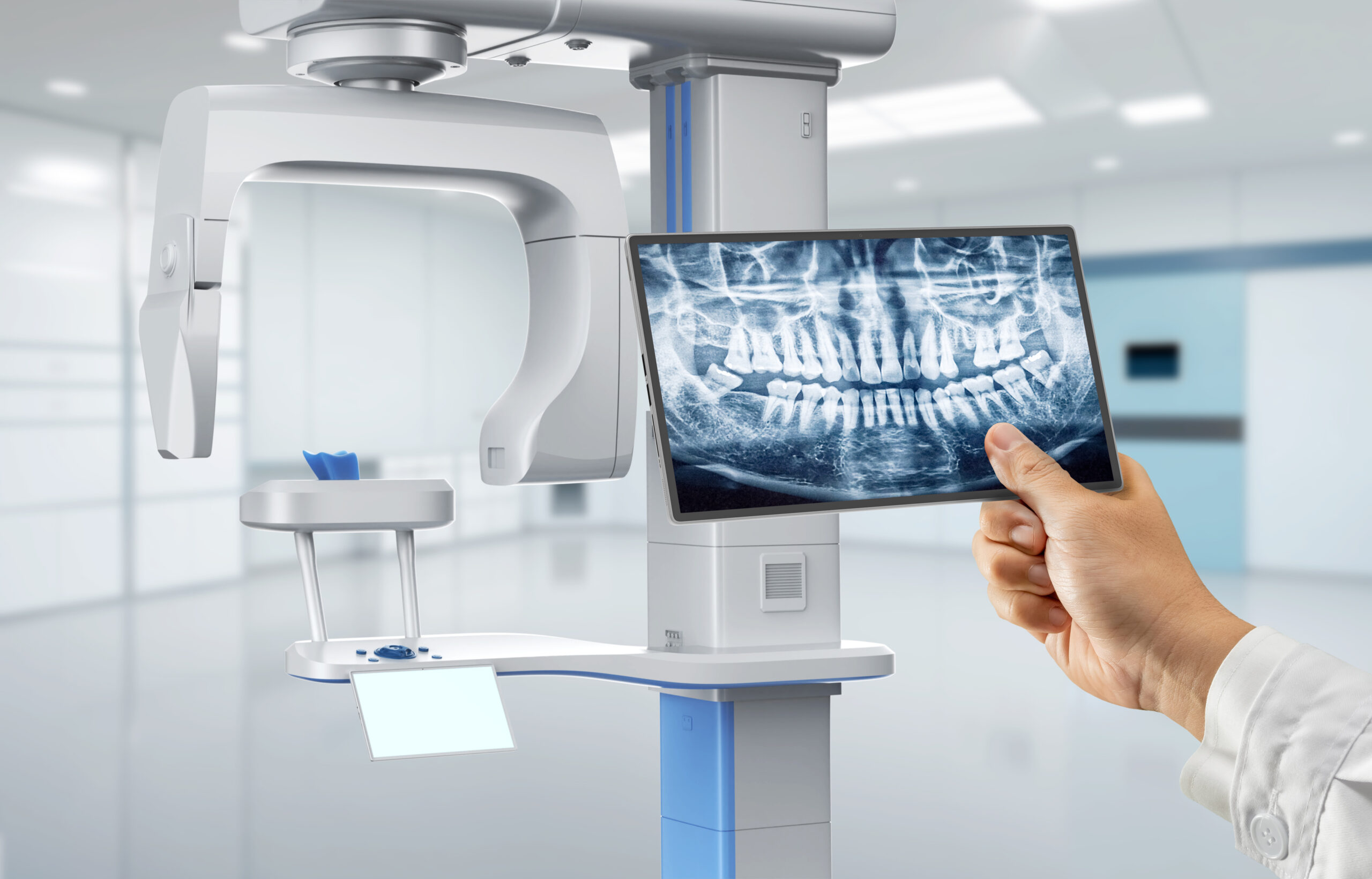
Digital dental radiography has transitioned from a premium option to a non-negotiable cornerstone of contemporary dental practice. Its criticality stems from three convergent imperatives: clinical efficacy, operational efficiency, and regulatory compliance. Modern intraoral and panoramic/CBCT systems reduce radiation exposure by 50-90% compared to film (adhering strictly to ALARA principles), while delivering superior diagnostic resolution through advanced sensor technology and AI-enhanced image processing. Crucially, these systems integrate natively with dental practice management software (PMS), CAD/CAM workflows, and cloud-based imaging archives, enabling real-time collaborative diagnostics and eliminating analog-to-digital conversion bottlenecks. For clinics, this translates to 30% faster patient throughput, demonstrable reduction in retake rates, and enhanced patient communication through immediate visual diagnostics. From a compliance perspective, GDPR/HIPAA-compliant digital workflows with audit trails are now mandatory in EU/US markets, making legacy film systems clinically and legally obsolete.
Market Segmentation: Premium Global Brands vs. Value-Optimized Solutions
The digital radiography market bifurcates into two strategic segments: European/US premium brands (exemplified by Dentsply Sirona, Planmeca, and Carestream Dental) targeting high-end clinics prioritizing seamless ecosystem integration and clinical validation, and value-optimized Chinese manufacturers led by Carejoy addressing cost-conscious clinics and emerging markets. While premium brands command 40-60% price premiums justified by proprietary sensor technology and deep PMS integrations, Carejoy has disrupted the mid-tier segment through aggressive component sourcing, modular software licensing, and strategic partnerships with regional distributors. This segmentation is not merely price-driven; it reflects divergent value propositions aligned with clinic operational models and regional reimbursement structures.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical & Commercial Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Carestream) | Carejoy (Value Segment Leader) |
|---|---|---|
| Price Range (Intraoral System) | €18,500 – €28,000 (sensor + software + installation) | €6,200 – €9,800 (fully configured system) |
| Sensor Technology | Proprietary CCD/CMOS with <14μm pixel pitch; patented scintillators (CsI) | High-grade CMOS (16-18μm pixel pitch); industrial-grade Gd₂O₂S scintillators |
| Software Integration | Native integration with major PMS (e.g., Dentrix, Curve Dental); DICOM 3.0 certified; AI caries detection (FDA 510k) | Modular SDK for 3rd-party PMS; DICOM 3.0 compliant; optional AI module (CE marked) |
| Service Network | Direct technicians in 28 EU countries; 4-hour SLA in metro areas; 12-month response for rural | Distributor-dependent coverage; 24-72hr response (urban); remote diagnostics standard; 48hr parts shipping |
| Radiation Dose Efficiency | 0.05-0.10 mGy (ISO 10970 certified); real-time dose monitoring | 0.12-0.18 mGy (IEC 60601-2-54 compliant); dose tracking via software |
| Warranty & Support | 3-year comprehensive (parts/labor); software updates included | 2-year limited (sensor 1yr); software updates via subscription (€299/yr) |
| Ideal Customer Profile | Multi-location premium clinics; university hospitals; practices with >€500k annual imaging volume | Single-location clinics; emerging market expansions; high-volume public health facilities |
Strategic Implications for Dental Clinics & Distributors
Clinics must align equipment selection with their clinical volume, reimbursement model, and digital ecosystem maturity. Premium brands justify investment through reduced downtime (95%+ uptime SLA) and workflow synergies in complex restorative/CBCT workflows, yielding 18-24 month ROI in high-fee environments. Carejoy delivers compelling value for clinics in price-sensitive markets (e.g., Eastern Europe, LATAM) or those prioritizing rapid deployment, with 8-14 month ROI where per-scan reimbursement is ≤€15. Distributors should note Carejoy’s 35% higher margin structure versus global brands (22-28%), but factor in required technical training investment. Critically, both segments now meet essential regulatory thresholds; differentiation lies in operational resilience and total cost of ownership beyond acquisition price. As AI-driven diagnostics become standard by 2027, clinics selecting modular platforms (including Carejoy’s SDK architecture) will avoid costly hardware obsolescence.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Digital Dental X-Ray Equipment
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 4–8 mA; Fixed anode tube; Single-phase generator with analog timer control. Requires standard 110–120V AC supply. Max power consumption: 650W. | 70–90 kVp, 4–15 mA; Rotating anode tube with high-frequency inverter generator. Digital exposure control with AEC (Automatic Exposure Control). Operates on 110–240V AC, auto-switching; Max power consumption: 900W with energy-saving standby mode. |
| Dimensions | Wall-mounted or floor-standing; Approx. 110 cm (H) × 45 cm (W) × 35 cm (D). Tube head extends up to 60 cm; Weight: ~35 kg. Requires minimum 1.2 m² floor space. | Compact floor-standing with telescopic arm; Approx. 125 cm (H) × 40 cm (W) × 30 cm (D). Motorized 3D positioning arm with 180° horizontal and 90° vertical articulation. Weight: ~42 kg with counterbalance system. Space-optimized design; 1.0 m² recommended. |
| Precision | Manual positioning with mechanical locks; Repeatability tolerance ±3° angular deviation. Image resolution up to 3.6 lp/mm with standard CCD sensors. No real-time image guidance. | Motorized positioning with digital angle presets and laser alignment. Repeatability tolerance ±0.5°. Integrated real-time image preview with AI-assisted positioning feedback. Supports high-resolution CMOS sensors up to 20 lp/mm with dynamic range optimization. |
| Material | Exterior housing: Powder-coated steel and ABS plastic. Tube housing: Aluminum alloy with oil insulation. Arm joints: Reinforced polycarbonate with stainless steel bearings. | Full aluminum-magnesium alloy chassis with anti-microbial coating. Hermetically sealed tube housing with dielectric fluid cooling. Robotic-grade harmonic drive joints with carbon-fiber arm components for reduced inertia. |
| Certification | Complies with IEC 60601-1 (3rd Ed), IEC 60601-2-54, FDA 510(k) clearance (Class II), CE Marked (MDD), ISO 13485 manufacturing. Local radiation safety compliance per country-specific regulations. | Full compliance with IEC 60601-1 (3rd Ed + AMD1), IEC 60601-2-54 (4th Ed), FDA 510(k) with AI software addendum, CE Marked (MDR 2017/745), ISO 13485:2016, and UL/CSA 60601-1. Includes DICOM 3.0, HL7, and NEMA connectivity standards certification. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Digital Dental X-Ray Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
1. Verifying ISO/CE Credentials: The Non-Negotiable Foundation
2026 Regulatory Context: EU MDR (2017/745) compliance is now mandatory for all CBCT and digital sensors. FDA 510(k) equivalence remains critical for U.S. market access. Chinese manufacturers must demonstrate adherence to ISO 13485:2016 with specific annexes for radiological devices.
Action Protocol:
- Request Original Certificates: Demand scanned copies with visible certification body seal (e.g., TÜV SÜD, BSI, SGS). Verify validity via certification body’s online portal.
- Check Scope of Approval: Certificate must explicitly list:
– “Dental Cone Beam Computed Tomography (CBCT)” OR “Intraoral Digital X-Ray Sensors”
– Specific model numbers covered under the certification - Validate CE Technical Documentation: Confirm manufacturer maintains full EU Technical File per Annex II/III of MDR. Red Flag: Generic “CE” stickers without 4-digit notified body number (e.g., CE 0123).
- 2026 Requirement: Confirm compliance with IEC 60601-2-63:2022 (amendment for dental X-ray equipment safety).
| Credential Type | Verification Method | 2026 Critical Checkpoints |
|---|---|---|
| ISO 13485:2016 | Check certificate # via IAF CertSearch | Must include “design and manufacturing of radiological dental equipment” in scope |
| EU CE Marking | Validate NB number on EUDAMED | MDR-compliant Technical File available upon request (per Article 29) |
| FDA Registration | Check Establishment # in FDA FURLS | 510(k) clearance for specific sensor/CBCT models (K-number required) |
2. Negotiating MOQ: Strategic Volume Planning for 2026
Market Shift: Post-pandemic supply chain normalization has increased MOQ flexibility, but semiconductor shortages persist for CMOS sensors. Distributors now leverage tiered pricing models while clinics benefit from “starter bundles”.
Optimization Strategies:
- Clinics: Target suppliers offering 5-unit MOQ for CBCT systems (2026 industry benchmark). Negotiate package deals (e.g., 1 CBCT + 2 sensors = 3-unit MOQ).
- Distributors: Qualify for container-load pricing (12-15 CBCT units or 30+ sensors) with flexible payment terms. Demand model-specific MOQs – avoid blanket minimums.
- 2026 Leverage Points:
- Commit to multi-year agreements for 15-20% MOQ reduction
- Negotiate “phantom inventory” – pay for units held at supplier until needed
- Request free demo units (counted toward MOQ) for sales training
- Avoid: Suppliers requiring >20-unit MOQ for CBCT without volume discount structure.
3. Shipping Terms: DDP vs. FOB in 2026 Logistics Realities
2026 Cost Drivers: Shanghai port congestion surcharges (avg. $850/container), EU carbon border tax (CBAM) implications, and mandatory electronic customs declarations (ICS2 Phase 3).
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight forwarder Lower base price |
Buyer assumes all risk after cargo loaded Customs clearance complexity |
Only for experienced importers with established freight partners Requires ICS2-compliant data submission 4h pre-loading |
| DDP (Delivered Duty Paid) | Single all-in price No hidden port fees |
Supplier manages all logistics/risk Guaranteed delivery timeline |
STRONGLY RECOMMENDED for 2026 Essential for EU compliance (CBAM calculation handled by supplier) |
Critical Shipping Checklist:
- Confirm supplier includes radiation shielding certification in shipping docs (required by IATA for X-ray equipment)
- Require real-time IoT tracking with temperature/humidity monitoring (critical for sensor calibration)
- Verify customs classification code (HS 9022 19 00 for dental CBCT) to avoid tariff errors
Verified Partner Profile: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Authority: ISO 13485:2016 (TÜV SÜD #1671381) with explicit scope for CBCT (CJ-9000 Series) and sensors. Full MDR Technical Files available.
- MOQ Flexibility:
- Clinics: 3-unit MOQ for CBCT (2026 Starter Program)
- Distributors: Tiered pricing from 8 units (CBCT) / 20 units (sensors) with container-load discounts
- Shipping Excellence:
- DDP standard for EU/US (includes CBAM compliance)
- Baoshan District factory: Direct rail link to Yangshan Port (avg. 72h Shanghai departure)
- IoT-enabled shipments with real-time radiation safety monitoring
2026 Value-Add Services: Free regulatory update webinars, 120-day payment terms for distributors, and onsite technician training at Shanghai facility.
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: No. 1888, Jiangyang North Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects 2026 regulatory standards as of Q4 2025. Verify all requirements with local authorities. Shanghai Carejoy is cited as a verified compliant supplier based on 2025 third-party audit data (SGS Report #CN2025-DENT-8814). Always conduct independent due diligence.
© 2026 Global Dental Sourcing Consortium. For internal use by licensed dental distributors and clinic procurement officers only.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Digital Dental X-Ray Systems
1. What voltage requirements should be considered when installing digital dental X-ray equipment in 2026?
Modern digital dental X-ray systems are typically designed to operate on standard clinical power inputs. Most intraoral and panoramic units require a stable 110–120V AC, 60Hz supply in North America and 220–240V AC, 50Hz in Europe and many international markets. However, with the increasing integration of AI-driven imaging software and high-frequency generators, power stability and surge protection are critical. Clinics should ensure dedicated circuits with clean power (low EMI) and consider installing voltage regulators or uninterruptible power supplies (UPS) to protect sensitive sensors and control boards. Always verify voltage compatibility with the manufacturer before installation, especially when retrofitting older facilities.
2. How accessible are spare parts for digital X-ray systems, and what should distributors stock?
Spare parts availability is a key factor in minimizing equipment downtime. Leading OEMs (e.g., Carestream, Dentsply Sirona, Planmeca) maintain global logistics networks with regional distribution centers to ensure 48–72 hour delivery of critical components. Distributors are advised to stock high-failure-rate items such as:
| Component | Typical Lifespan | Recommended Stock Level (per 10 units) |
|---|---|---|
| X-ray Sensor Cables & Connectors | 12–18 months | 2–3 |
| Position Indicating Devices (PIDs) | 3–5 years | 1 |
| Tube Head Cooling Fans | 4–6 years | 1 |
| Control Panel Keypads | 5+ years | 1 |
Additionally, verify that the manufacturer offers long-term parts support (minimum 7–10 years post-discontinuation) and provides firmware-compatible replacement modules.
3. What does the installation process for digital X-ray systems involve, and is professional setup required?
Yes, professional installation by a certified biomedical technician or OEM-trained engineer is mandatory for compliance, safety, and optimal performance. The installation process in 2026 includes:
- Site assessment (structural support, wall mounting, power quality, network connectivity)
- Radiation shielding verification (compliance with local regulations such as NCRP Report No. 177)
- Hardware mounting (ceiling-suspended, floor-standing, or wall-mounted units)
- Network integration with DICOM 3.0 and clinic PMS (Practice Management Software)
- Calibration and sensor alignment using test phantoms
- Staff training on operation, infection control, and emergency protocols
Most manufacturers include turnkey installation in the purchase agreement for premium models. Distributors should confirm that installation services are available locally or through authorized partners before quoting.
4. What warranty terms are standard for digital dental X-ray equipment in 2026?
The industry standard for digital X-ray systems in 2026 includes a 2-year comprehensive warranty covering parts, labor, and on-site service for manufacturing defects. Premium-tier systems may offer extended coverage up to 3–5 years, particularly for the X-ray tube and digital sensors. Key warranty considerations include:
| Component | Standard Warranty | Extended Options |
|---|---|---|
| X-ray Generator & Tube | 2 years | Optional 3rd year (pro-rata) |
| Digital Sensors (CMOS/CCD) | 1–2 years (non-mechanical damage) | Extended to 3 years with service plan |
| Control Electronics | 2 years | Included in full-system coverage |
| Software & AI Modules | Lifetime updates (cloud-dependent) | Annual support subscription recommended |
Warranties are typically voided by unauthorized repairs, power surges, or improper use. Distributors should emphasize the importance of registering equipment within 30 days and maintaining service logs.
5. Are post-warranty service and spare parts guaranteed beyond the initial coverage period?
Reputable manufacturers guarantee spare parts and technical support for a minimum of 7 years after product discontinuation, in alignment with ISO 13485 and medical device lifecycle standards. However, clinics and distributors must plan strategically:
- Confirm End-of-Life (EOL) notification policy (typically 18–24 months in advance)
- Enroll in post-warranty service contracts that include priority response, predictive maintenance, and discounted parts
- Verify backward compatibility of new sensors and software with legacy systems
- Consider refurbishment programs or trade-in options for aging units
Distributors should maintain close coordination with OEM service networks to ensure continuity of support and offer clients lifecycle management packages as part of equipment procurement.
© 2026 Professional Dental Equipment Guide | For B2B Use – Not for Consumer Distribution
Need a Quote for Digital Dental X Ray Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


