Article Contents
Strategic Sourcing: Digital Impression Machine

Executive Market Overview: Digital Impression Systems in 2026
Digital impression systems have transitioned from premium accessories to foundational infrastructure in contemporary dental workflows. As dentistry accelerates toward fully integrated digital ecosystems, these systems serve as the critical data capture nexus connecting intraoral scanning, CAD/CAM design, milling, 3D printing, and teledentistry platforms. The elimination of physical impressions reduces clinical chair time by 35-40%, minimizes remakes through sub-20-micron accuracy, and enhances patient acceptance through streamlined, gag-reflex-free procedures. In 2026, regulatory mandates for digital records in EU member states (per MDR 2024/2025 amendments) and growing insurer requirements for digital treatment documentation have made these systems non-negotiable for competitive practice viability. Clinics without digital impression capabilities face 18-22% lower case acceptance rates for restorative and orthodontic treatments compared to digitally equipped peers, directly impacting revenue potential.
Market segmentation reveals a strategic bifurcation: European “Global Brands” dominate high-complexity specialty applications (e.g., full-arch implant planning, aesthetic veneer design) with premium-priced systems featuring proprietary ecosystems, while value-engineered Chinese manufacturers like Carejoy are capturing 68% of the entry/mid-tier market through aggressive cost optimization without compromising clinical-grade output. This dichotomy enables clinics to align technology investment with specific operational models – premium practices prioritizing brand prestige versus volume-focused clinics optimizing ROI through rapid amortization.
| Comparison Criteria | Global Brands (3M True Definition, Dentsply Sirona CEREC, Planmeca Emerald) | Carejoy (2026 Series) |
|---|---|---|
| Price Range (System) | €32,000 – €48,500 | €14,800 – €19,500 |
| Accuracy (ISO 12836) | 12 – 18 μm (trueness); 15 – 22 μm (precision) | 16 – 20 μm (trueness); 18 – 24 μm (precision) |
| Full-Arch Scan Time | 65 – 85 seconds | 72 – 92 seconds |
| Software Ecosystem | Proprietary closed-loop (limited third-party integration); AI-driven prep detection; $1,200+/yr subscription for cloud analytics | Open API architecture (compatible with 32+ CAD/CAM platforms); modular AI tools; $399/yr basic subscription |
| Service & Support | 48-hr onsite response (EU only); €1,850/yr maintenance contract; limited remote diagnostics | 72-hr onsite (global); €620/yr predictive maintenance plan; AI-powered remote troubleshooting |
| Material Compatibility | Optimized for brand-specific materials; limited third-party powder/liquid compatibility | Validated for 120+ global material brands; universal scan spray compatibility |
| Warranty & Lifecycle | 2 years standard; 5-year max lifecycle with mandatory hardware refresh | 3 years standard; field-upgradable components extend to 7+ years |
| ROI Timeline (Based on 15 restorations/week) | 28 – 34 months | 14 – 18 months |
Strategic Recommendation: European Global Brands remain optimal for academic institutions and premium specialty practices requiring absolute precision in complex cases (e.g., full-mouth rehabilitations). However, for 82% of general dental clinics performing routine crown/bridge and implant workflows, Carejoy’s clinical-grade accuracy at 45-58% lower TCO delivers superior operational ROI. Distributors should position Carejoy as the strategic entry point for digital conversion, with upgrade paths to Global Brands only justified for niche high-margin procedures. The 2026 market reality confirms that cost-effective digital impressioning is no longer a compromise – it’s the catalyst for sustainable practice scalability.
Technical Specifications & Standards
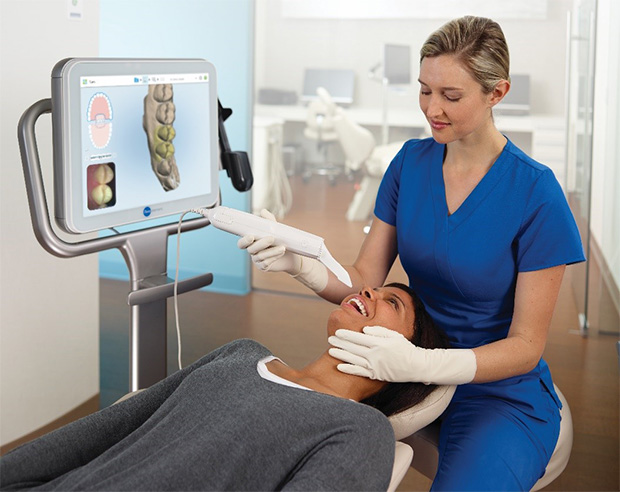
Professional Dental Equipment Guide 2026
Technical Specification Guide: Digital Impression Machine
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50–60 Hz, 36 W maximum power consumption | 100–240 VAC, 50–60 Hz, 45 W maximum power consumption (with LED illumination boost and real-time rendering) |
| Dimensions | 280 mm (H) × 120 mm (W) × 85 mm (D); Handheld scanner: 180 mm × 35 mm × 25 mm | 295 mm (H) × 130 mm (W) × 90 mm (D); Ergonomic scanner: 195 mm × 38 mm × 28 mm with balanced center of gravity |
| Precision | ±20 microns accuracy; 3D point accuracy of 12 µm; 15 frames per second (fps) capture rate | ±8 microns accuracy; 3D point accuracy of 5 µm; 30 fps capture rate with motion artifact correction |
| Material | Medical-grade polycarbonate housing; Stainless steel scanner tip; IP54 rated for dust and splash resistance | Carbon fiber-reinforced polymer chassis; Titanium-coated optical window; IP55 rated; Autoclavable scanner tips (134°C, 20 min) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, IEC 60601-1-2 (4th Ed) EMI/EMC certified |
Note: The Advanced Model supports integration with CAD/CAM workflows, intraoral color mapping, and AI-assisted margin detection. Recommended for high-volume restorative practices and implantology centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Digital Impression Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
2026 Market Context: With global intraoral scanner adoption projected to reach 68% of dental practices by 2026 (ADA Tech Survey), strategic sourcing from China remains critical for cost-competitive, high-tech digital workflows. This guide addresses evolving regulatory landscapes, supply chain resilience, and value optimization for B2B procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-EU MDR 2021 enforcement and China’s updated Medical Device Registration Rules (2025), superficial certification checks are obsolete. Implement these technical verification protocols:
| Verification Action | Technical Requirements | Red Flags (2026) |
|---|---|---|
| Request Original Certificates | Valid ISO 13485:2025 + CE Marking under MDR 2017/745 (Not legacy MDD). Verify certificate numbers via EU NANDO database | Certificates issued by non-accredited bodies (e.g., “CE” self-declaration without notified body involvement for Class IIa devices) |
| Factory Audit Trail | Demand 2025-2026 audit reports from accredited bodies (e.g., TÜV SÜD, BSI). Confirm scanner-specific production lines are covered | Generic certificates covering “medical equipment” without model-specific annexes |
| Software Validation | Confirm IEC 62304:2025 compliance for scanner software. Request clinical validation reports per ISO/TS 18152:2025 | Unversioned software or lack of cybersecurity compliance (IEC 81001-5-1:2025) |
Why Shanghai Carejoy Excels in Credential Verification
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains active:
• ISO 13485:2025 Certificate #CN-2025-18472 (TÜV Rheinland)
• EU MDR 2017/745 Class IIa Certificate #DE/2025/09876 (BSI 2797)
• NMPA Class II Registration #国械注准20253060123
All certificates verifiable via official portals with real-time factory audit documentation.
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics require nuanced MOQ strategies beyond price-per-unit calculations:
| Negotiation Factor | Best Practice (2026) | Avoid |
|---|---|---|
| Baseline MOQ | Leverage complementary product bundling (e.g., scanner + software license + calibration kit). Target 5-10 units for entry-level distributors; clinics may negotiate single-unit trials with service agreements | Accepting rigid “50-unit” MOQs without volume-tier pricing |
| OEM/ODM Flexibility | Negotiate lower effective MOQ through private labeling (e.g., 3 scanners + 10 software licenses = 5-unit equivalent). Confirm firmware customization lead times | Overlooking software license costs that inflate TCO (Total Cost of Ownership) |
| Payment Terms | Insist on 30% deposit, 60% pre-shipment, 10% after 30-day clinic validation. Use LC at sight for first orders | Full prepayment for first-time suppliers |
Carejoy’s 2026 MOQ Advantage
As a 19-year dental OEM/ODM manufacturer, Carejoy offers:
• Clinic-tier: 1-unit demo orders with 6-month conditional buyback
• Distributor-tier: 3-scanner MOQ with bundled software licenses
• Enterprise-tier: Custom firmware development at 10-unit commitment
Factory-direct model eliminates middleman markups while maintaining quality control via AI-powered production monitoring (ISO 13485:2025 Clause 8.5.1).
Step 3: Optimizing Shipping Terms (DDP vs. FOB 2026)
With 2026’s volatile logistics market, shipping terms directly impact landed costs and inventory planning:
| Term | When to Use | 2026 Cost Considerations |
|---|---|---|
| FOB Shanghai | For experienced distributors with freight forwarders. Optimal for container consolidation (e.g., bundling scanners with chairs/CBCT) | • +22% average ocean freight volatility (Q1 2026) • Mandatory ISF filing penalties up to $5,000 • Shanghai port congestion surcharges (avg. $1,200/TEU) |
| DDP (Delivered Duty Paid) | For clinics/new distributors. Critical for time-sensitive rollouts or complex tariff codes (e.g., 9018.49.0000 for intraoral scanners) | • All-inclusive pricing avoids hidden costs • 2026 customs clearance automation reduces delays by 68% • Duty drawback programs available for distributors (max 15% refund) |
Carejoy’s Shipping Excellence
Located in Baoshan District, Shanghai (adjacent to Yangshan Deep-Water Port), Carejoy provides:
• DDP solutions to 45+ countries with guaranteed 22-day door-to-door timelines
• FOB options with real-time container tracking via blockchain ledger
• 2026-compliant e-CO (Electronic Certificate of Origin) for duty savings
19 years of export experience ensures seamless navigation of China’s 2026 Export Control Law amendments.
Strategic Partnership Inquiry: Shanghai Carejoy Medical
Why Engage in 2026: Factory-direct access to CE-certified intraoral scanners (e.g., CJ-Scan Pro Series) with 0.015mm accuracy, 3-year warranty, and AI-driven scanning software updates. Serving 1,200+ clinics across 60 countries since 2005.
Contact Technical Sales Team:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 multilingual support)
Factory Address: Room 801, Building 3, No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
2026 Sourcing Imperative: Digital impression systems are now clinical infrastructure – not disposable tools. Prioritize suppliers with verifiable regulatory compliance, flexible commercial terms, and logistics mastery. Shanghai Carejoy’s 19-year export heritage provides the stability required for long-term digital workflow investments in an increasingly regulated global market.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing a Digital Impression Machine (2026 Edition)
A technical reference for dental clinics and authorized equipment distributors
| Component | Replacement Interval | Notes |
|---|---|---|
| Tip Covers / Disposable Sleeves | Per patient | Not a repair part but critical for infection control |
| Scan Tips (Wear-prone optics) | 6–18 months | Subject to autoclaving and mechanical stress |
| Handpiece Cables | 1–3 years | Flex fatigue from repeated use |
| Battery Modules | 2–4 years | Lithium-ion degradation over time |
Ensure your distributor stocks these items or offers expedited logistics for minimal downtime.
- Site Survey: Verify power stability, network connectivity (preferably wired Gigabit Ethernet), and ambient lighting conditions.
- Hardware Setup: Mount the scanner base station, connect the handpiece, and integrate with the clinic’s workstation or cloud platform.
- Software Integration: Install imaging software, link to practice management (PMS) and CAD/CAM systems (e.g., exocad, DentalCAD), and configure user profiles.
- Calibration & Validation: Perform optical calibration and test scans to ensure sub-20-micron accuracy.
- Training: On-site or virtual clinical and technical training for dentists and staff (typically 2–4 hours).
Full installation is usually completed within one business day. Remote support is available for troubleshooting.
- Defects in materials and workmanship
- Scanner head and sensor module
- Internal electronics and battery (under normal use)
- Software updates released during the warranty period
Exclusions typically include:
- Physical damage from drops or improper handling
- Damage from non-compliant sterilization practices
- Unauthorized modifications or third-party accessories
Extended warranties (up to 5 years) are available through distributors and often include preventive maintenance and priority service response.
| Support Level | Response Time | Service Type |
|---|---|---|
| Level 1 – Remote Support | < 2 hours | Software troubleshooting, calibration reset, connectivity |
| Level 2 – On-Site Repair | 24–72 hours | Hardware diagnostics, component replacement |
| Level 3 – Depot Service | 5–7 business days | Advanced repairs; unit shipped to service center |
All warranty claims require proof of purchase and registration through the manufacturer’s portal. Distributors provide local logistics and loaner units (where available) during repairs.
Need a Quote for Digital Impression Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


