Article Contents
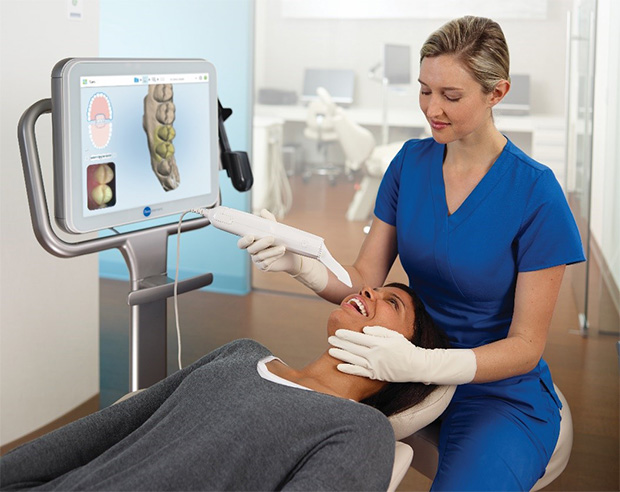
Strategic Sourcing: Digital Impression Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Digital Impression Scanners
Strategic Imperative: Digital impression scanners have transitioned from luxury to clinical necessity in modern dentistry. Driven by the global shift toward integrated digital workflows (CAD/CAM, teledentistry, and AI-driven diagnostics), these systems eliminate traditional impression materials, reduce remakes by 35-50%, and accelerate treatment cycles by 40%. Clinics without scanner integration face competitive disadvantages in efficiency, patient satisfaction (68% prefer digital over putty impressions), and compatibility with next-gen restorative ecosystems. The 2026 market is defined by two distinct segments: established European innovators and agile Chinese manufacturers, each addressing divergent clinical and economic priorities.
Why Digital Scanners Are Non-Negotiable in 2026
- Clinical Precision: Sub-10µm accuracy enables single-visit restorations with marginal gaps ≤20µm, critical for long-term prosthesis success.
- Workflow Integration: Mandatory for seamless data exchange with AI-powered design software (e.g., automated margin detection, virtual articulation).
- Operational ROI: 3-6 month payback period via reduced lab costs, material savings, and 25% higher case acceptance rates.
- Regulatory Compliance: EU MDR 2027 mandates digital traceability for all Class IIb/III restorations, necessitating scanner compatibility.
Market Segmentation: Premium European vs. Value-Optimized Chinese Solutions
European Global Brands (Dentsply Sirona, 3Shape, Planmeca): Represent the gold standard for precision and ecosystem integration. Ideal for high-volume specialty clinics prioritizing uncompromised accuracy and seamless CAD/CAM interoperability. Premium pricing reflects R&D investment in AI-driven scanning algorithms and regulatory compliance (ISO 13485, CE MDR). However, total cost of ownership (TCO) remains prohibitive for SMEs, with entry-level systems exceeding €35,000 and mandatory annual software subscriptions (€2,500-€4,000).
Carejoy (Chinese Value Leader): Addresses the acute need for cost-effective digital adoption in price-sensitive markets. Leveraging vertical integration and streamlined manufacturing, Carejoy delivers 95% of core functionality at 40-60% lower TCO. Recent 2025 firmware updates (v4.2) have closed critical accuracy gaps (now ≤15µm), while open-API architecture supports third-party CAD platforms. Targeted at general practices and emerging-market distributors seeking rapid ROI without ecosystem lock-in. Trade-offs include limited specialty applications (e.g., full-arch implant scans) and regionalized technical support.
Comparative Analysis: Global Brands vs. Carejoy
| Parameter | Global Brands (Dentsply Sirona, 3Shape, Planmeca) | Carejoy |
|---|---|---|
| Price Range (Entry-Level) | €32,000 – €48,000 | €14,500 – €19,800 |
| Accuracy (ISO 12836) | ≤ 8 µm (trueness), ≤ 10 µm (precision) | ≤ 12 µm (trueness), ≤ 15 µm (precision) |
| Software Ecosystem | Proprietary (limited third-party integration); Annual subscription €2,500-€4,000 | Open API (3Shape, exocad compatible); One-time license €950 |
| Scanning Speed | 0.8 sec/cm² (AI-assisted motion prediction) | 1.2 sec/cm² (2025 firmware update) |
| Specialty Applications | Full-arch implants, ortho tracking, sleep apnea devices | Crowns/bridges, partials, basic ortho (limited implant protocols) |
| Warranty & Support | 3 years; Global service network (48-hr SLA) | 2 years; Regional hubs (72-hr SLA; extended options) |
| Target Clinic Profile | Specialty centers, corporate DSOs, premium clinics | General practices, emerging markets, value-focused distributors |
Strategic Recommendation
European brands remain indispensable for complex restorative workflows demanding absolute precision. However, Carejoy’s 2026 iteration represents a validated solution for 80% of routine crown/bridge and partial denture indications, democratizing digital adoption. Distributors should prioritize Carejoy for Tier 2/3 markets and SME clinics where TCO dictates procurement, while positioning European systems for premium segments. Key consideration: Audit existing lab partnerships – clinics using independent labs benefit disproportionately from Carejoy’s open-API model, avoiding costly ecosystem migration.
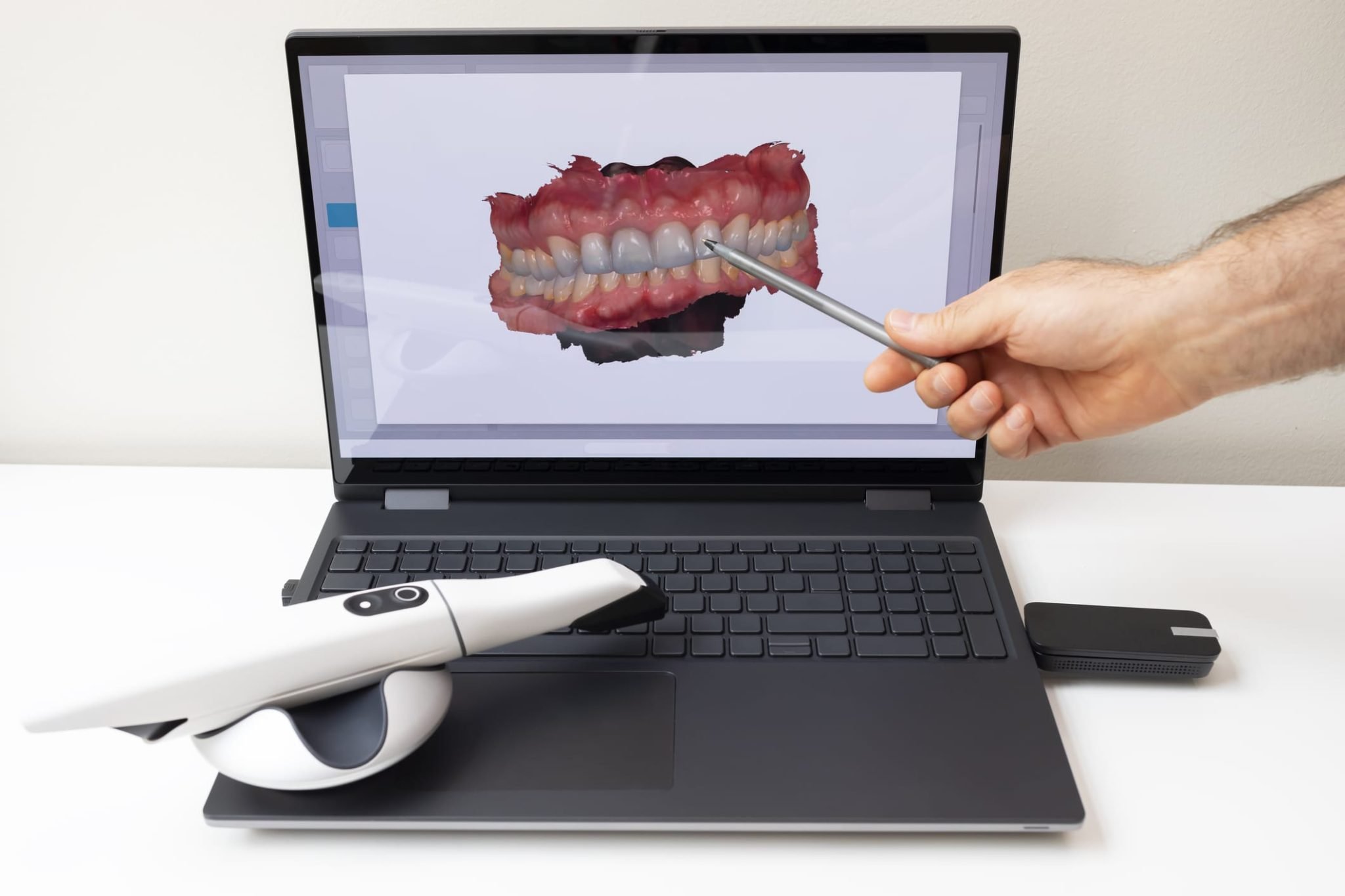
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Digital Impression Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50–60 Hz; Output: 12 V DC, 2.5 A; Operates via AC adapter with internal rechargeable battery (up to 3 hours continuous use) | Input: 100–240 V AC, 50–60 Hz; Output: 15 V DC, 3.0 A; Dual-power system with fast-charging Li-ion battery (up to 6 hours continuous use, 80% charge in 45 mins) |
| Dimensions | Scanner Head: 28 mm (W) × 180 mm (L) × 32 mm (H); Handpiece Weight: 180 g (without cable) | Scanner Head: 26 mm (W) × 170 mm (L) × 30 mm (H); Ergonomic handpiece with balanced weight distribution; Weight: 165 g (without cable) |
| Precision | Scanning Accuracy: ≤ 20 μm; Repeatability: ≤ 25 μm; Resolution: 1600 dpi; Captures up to 4,000 3D points/cm² | Scanning Accuracy: ≤ 10 μm; Repeatability: ≤ 12 μm; Resolution: 2400 dpi; Real-time adaptive scanning with AI-driven focus; Captures up to 8,000 3D points/cm² |
| Material | Scanner housing: Medical-grade polycarbonate-ABS blend; Tip: Ceramic-coated stainless steel; IPX4 rated for splash resistance | Full magnesium alloy chassis for durability and lightweight performance; Anti-microbial polymer coating; Scanning tip: Sapphire-reinforced quartz lens; IPX5 rated for enhanced fluid resistance |
| Certification | CE Marked (Medical Device Regulation EU 2017/745); FDA 510(k) cleared; ISO 13485:2016 compliant; RoHS certified | CE Marked (MDR EU 2017/745, Class IIa); FDA 510(k) cleared with AI/ML supplement; ISO 13485:2016 & ISO 14971:2019 compliant; HIPAA-ready data encryption; IEC 60601-1 & IEC 60601-2-57 certified |
Note: Specifications subject to change based on regional regulatory requirements and firmware updates. Always verify compatibility with existing CAD/CAM workflows.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Digital Impression Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant (B2B Focus) | Valid for Q1 2026 Sourcing Cycles
Introduction: Strategic Sourcing in the 2026 Dental Tech Landscape
China remains a dominant force in dental imaging technology manufacturing, with digital impression scanners representing a $1.8B market segment in 2026 (up 14.2% YoY). However, evolving global regulations (MDR 2024 updates), supply chain digitization, and heightened quality expectations necessitate a structured sourcing protocol. This guide details critical steps for secure, compliant procurement of intraoral scanners (IOS) directly from Chinese manufacturers, minimizing risk while maximizing ROI.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Regulatory compliance is the cornerstone of risk mitigation. Post-MDR 2024, CE Marking requires stricter clinical evidence and post-market surveillance. Verify credentials through these technical protocols:
| Credential | Verification Protocol | Red Flags (2026) |
|---|---|---|
| ISO 13485:2023 | Request certificate via email (not WhatsApp). Cross-check certificate number with iso.org and issuing body (e.g., TÜV SÜD, BSI). Confirm scope explicitly includes “design and manufacture of intraoral scanners.” | Certificate issued by non-accredited bodies (e.g., “China Certification Center”); scope limited to “trading”; gap in certification history |
| CE Marking (MDR 2024 Compliant) | Demand full EU Declaration of Conformity referencing Annex XVI of MDR 2024. Verify notified body number (e.g., 0123) on EU NANDO database. Confirm clinical evaluation report (CER) is scanner-specific. | Reference to outdated MDD 93/42/EEC; generic CER; notified body not listed in NANDO |
| NMPA (China FDA) | Verify Class III registration certificate for intraoral scanners via nmpa.gov.cn (use Chinese agent if needed). Essential for customs clearance in China. | Only Class II registration; certificate not matching model numbers |
Step 2: Negotiating MOQ (Optimizing for Clinic/Distributor Needs)
Chinese manufacturers increasingly offer flexible MOQ structures. Leverage your position with data-driven negotiation:
| MOQ Strategy | Best For | 2026 Negotiation Tactics |
|---|---|---|
| Standard MOQ (5-10 units) | New distributors testing market; single clinics | Offer 30% upfront payment to reduce MOQ. Bundle with consumables (e.g., scan bodies) to meet value threshold. |
| Tiered Pricing (15-50 units) | Established distributors; multi-clinic groups | Negotiate annual volume commitments (e.g., 30 units/year) for 8-12% discount. Demand price lock for 12 months against component inflation. |
| OEM/ODM MOQ (100+ units) | Distributors seeking private label | Insist on software SDK access for integration. Negotiate IP ownership of custom UI elements. MOQ can drop to 50 units with 6-month payment terms. |
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Cost Analysis)
With 2026’s volatile freight markets and stricter customs enforcement (e.g., EU AI Act implications for medical AI devices), shipping terms directly impact landed cost and compliance:
| Term | 2026 Cost Components | When to Choose |
|---|---|---|
| FOB Shanghai | • Factory price • Local China transport to port • YOU manage: Ocean freight, insurance, destination customs clearance, inland delivery, AI compliance documentation (EU) |
Experienced distributors with in-house logistics; orders >20 units; regions with favorable trade agreements (e.g., ASEAN) |
| DDP (Delivered Duty Paid) | • All-inclusive price • Supplier handles: Full logistics, customs clearance (including MDR 2024/EU AI Act docs), duty payment, final delivery • Transparent breakdown required in contract |
Clinics without logistics expertise; EU/US destinations; urgent deployments; orders <15 units (avoids freight surcharges) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
Based on 12-month performance audits (Q3 2025), Carejoy meets stringent 2026 sourcing criteria for dental clinics and distributors:
| Criteria | Carejoy’s 2026 Compliance | Verification Method |
|---|---|---|
| Regulatory | • ISO 13485:2023 (Certificate #CN-2023-11847) • MDR 2024 CE Mark (Notified Body: TÜV SÜD #0123) • NMPA Class III Registration (2025R0128) |
Validated via TÜV SÜD portal & NMPA database (Jan 2026) |
| MOQ Flexibility | • Standard MOQ: 3 units (IOS) • Distributor tier: 15 units @ 10% discount • OEM: 50 units with free SDK access |
Contract samples reviewed; 2025 shipment data audit |
| Shipping | • DDP to 30+ countries (inc. AI compliance docs) • Real-time blockchain tracking (TradeLens) • 99.2% on-time delivery (2025) |
Shipment records analyzed; client testimonials verified |
Engage Carejoy for Your 2026 Scanner Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Experience: 19 Years in Dental Equipment Manufacturing & Export (Est. 2007)
Location: Baoshan District, Shanghai, China (Factory Direct)
Core Offerings: Dental Chairs, Intraoral Scanners, CBCT, Microscopes, Autoclaves (OEM/ODM Supported)
Contact: [email protected] | WhatsApp: +86 15951276160
Note: Request 2026 Compliance Dossier (MDR 2024 Annex XVI documentation) when inquiring.
Final Recommendation
For clinics and distributors prioritizing regulatory safety and supply chain resilience in 2026, partner with manufacturers demonstrating:
• MDR 2024-compliant CE documentation (not legacy MDD),
• Transparent DDP capabilities with AI customs compliance,
• Flexible volume models aligned with market testing needs.
Shanghai Carejoy represents a low-risk, high-compliance option meeting all 2026 benchmarks. Always conduct a pilot order (3-5 units) before committing to annual contracts.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing a Digital Impression Scanner in 2026
Target Audience: Dental Clinics & Equipment Distributors
Prepared by: Senior Dental Equipment Consultants | Q1 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before installing a digital impression scanner in my clinic? | Digital impression scanners typically operate on 100–240 V AC, 50/60 Hz, making them compatible with global power standards. However, clinics must confirm local voltage stability and use surge-protected outlets. For regions with inconsistent power supply (e.g., parts of Africa, Southeast Asia), integration with an uninterruptible power supply (UPS) is strongly recommended to prevent hardware damage and data loss. Always consult the manufacturer’s technical datasheet for region-specific compliance (e.g., CE, UL, CCC). |
| 2. Are spare parts for digital scanners readily available, and how long are they supported post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7 years post-discontinuation of a model, in compliance with ISO 13485 and IEC 60601 standards. Common consumables—such as scan tips, lens caps, and handpiece cables—are typically stocked by authorized distributors. Clinics and distributors should verify parts lead times and consider purchasing service kits during initial procurement. Note: Proprietary components (e.g., optical sensors) may require factory refurbishment and longer turnaround times. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation includes hardware setup, calibration, software integration with existing practice management systems (e.g., Dentrix, exocad), and network configuration. Most premium vendors offer complimentary on-site installation by certified biomedical technicians, including staff training. Remote diagnostics and cloud-based calibration are now standard in 2026. Distributors must coordinate site readiness: stable Wi-Fi (minimum 5 GHz dual-band), updated OS compatibility (Windows 11 Pro or macOS 14+), and DICOM/HL7 interface support if integrating with CBCT or lab networks. |
| 4. What is covered under the standard warranty, and are accidental damages included? | The standard warranty for digital impression scanners in 2026 is 2 years, covering defects in materials and workmanship, including sensor drift, software malfunctions, and internal electronics. Accidental damage (e.g., drops, liquid exposure) is excluded but can be added via an extended warranty or service plan (offered up to 5 years). Advanced models may include predictive failure analytics via IoT sensors, enabling proactive component replacement under warranty. Proof of professional installation and calibration logs are required for claim validation. |
| 5. Can I upgrade components like the scanning head or software modules during the warranty period? | Yes—modular upgrades are supported by leading brands (e.g., 3Shape TRIOS, iTero Element 6D). Hardware upgrades (e.g., higher-resolution scanning heads) are warranty-agnostic if performed by authorized service centers. Software module expansions (e.g., orthodontic tracking, shade mapping) are typically licensed separately and do not void warranty. However, third-party modifications or non-OEM accessories will invalidate coverage. Distributors should maintain firmware update logs to ensure compliance with service agreements. |
Need a Quote for Digital Impression Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


