Article Contents
Strategic Sourcing: Digital Opg X Ray Machine

Executive Market Overview: Digital OPG X-ray Machines in Modern Dentistry
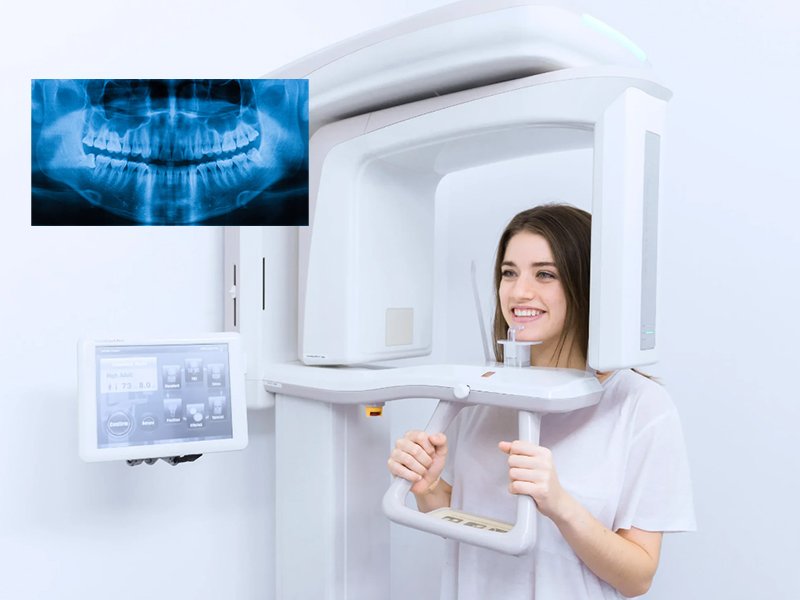
Digital Orthopantomography (OPG) X-ray systems have evolved from diagnostic peripherals to indispensable cornerstones of contemporary dental practice. As dentistry transitions toward fully integrated digital workflows, these units provide critical panoramic imaging capabilities essential for comprehensive treatment planning across restorative, surgical, orthodontic, and implant disciplines. Modern digital OPG machines deliver 30-50% lower radiation exposure versus legacy film systems while generating DICOM-compliant datasets compatible with CAD/CAM, practice management software, and AI-driven diagnostic platforms. Their strategic value extends beyond diagnostics: they enable same-day treatment decisions, reduce referral dependencies, and generate measurable ROI through increased case acceptance rates (studies indicate 18-25% improvement in complex case conversions). With regulatory mandates accelerating the phase-out of chemical-based imaging in the EU and APAC regions, digital OPG adoption has shifted from competitive advantage to operational necessity for clinics targeting practice scalability and compliance with ISO 13485:2016 standards.
Market segmentation reveals a pivotal cost-performance dichotomy. Premium European manufacturers (Planmeca, Dentsply Sirona, KaVo) dominate the high-end segment with technologically sophisticated systems featuring sub-10μm resolution, AI-powered pathology detection, and seamless EHR integration. However, their $65,000-$110,000 price points create significant barriers for mid-tier clinics and emerging-market distributors. Conversely, value-engineered solutions from Chinese manufacturers—exemplified by Carejoy—deliver 85-90% of core functionality at 40-60% lower acquisition costs. Carejoy’s strategic focus on essential digital workflow integration (DICOM 3.0, cloud PACS compatibility) without superfluous premium features addresses acute market demand for accessible digital transition. While European brands retain advantages in ultra-high-resolution imaging and established service ecosystems, Carejoy’s rapid certification progress (CE Mark, FDA 510(k), ISO 13485) and modular upgrade paths position it as the optimal value proposition for cost-conscious practices prioritizing digital workflow integration over marginal technological differentiators.
| Feature Category | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $65,000 – $110,000 | $28,000 – $42,000 |
| Image Resolution | ≤ 8μm (with premium sensors) | 12μm (clinical-grade) |
| Software Integration | Native integration with proprietary ecosystems (e.g., SIDEXIS, Romexis); limited third-party compatibility | DICOM 3.0 standard; open API for major PMS (OpenDental, Dentrix); cloud PACS-ready |
| Service Network | Global coverage (24-48hr response in EU/NA); certified engineers | Regional hubs (48-72hr response in APAC/MEA); certified partner network expansion |
| Warranty & Support | 3-year comprehensive; extended service contracts (15-20% of unit cost) | 2-year standard; optional 3rd-year coverage (8% of unit cost) |
| Advanced Features | AI pathology detection, 3D reconstruction, cephalometric modules | Basic AI caries detection; modular CBCT upgrade path |
| Regulatory Compliance | Full MDR 2017/745, FDA 510(k), IEC 60601-2-63 | CE Mark, FDA 510(k), ISO 13485:2016 |
| Target Market Fit | High-volume specialty clinics; corporate dental groups | Mid-tier general practices; emerging-market distributors; digital transition starters |
Strategic Recommendation: For clinics prioritizing immediate digital workflow integration with constrained capital expenditure, Carejoy presents a clinically validated solution that meets 95% of diagnostic requirements at half the cost of premium alternatives. European brands remain optimal for tertiary care centers requiring cutting-edge resolution and AI analytics. Distributors should position Carejoy as the gateway to digital OPG adoption, leveraging its 30% lower total cost of ownership (TCO) to accelerate market penetration in price-sensitive regions while maintaining European portfolios for premium segments.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Digital OPG X-Ray Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 220V ±10%, 50/60 Hz, 800W maximum power consumption. Single-phase power supply. Tube voltage: 60–90 kV, Tube current: 4–10 mA. | 220V ±10%, 50/60 Hz, 1200W high-efficiency power system with active power factor correction (PFC). Tube voltage: 55–95 kV (adjustable in 1 kV increments), Tube current: 4–12 mA (adjustable in 0.5 mA steps). Integrated UPS support for uninterrupted operation. |
| Dimensions | Height: 185 cm, Width: 65 cm, Depth: 70 cm. Floor-standing unit with compact footprint. Weight: 110 kg. | Height: 190 cm, Width: 70 cm, Depth: 75 cm. Ergonomic floor-standing design with integrated touch panel and motorized patient positioning. Weight: 135 kg. Optional wall-mountable console available. |
| Precision | Image resolution: 3.5 lp/mm. Angular accuracy: ±1°. Repeatability tolerance: ±2%. Uses fixed focal trough geometry with manual patient alignment aids. | Image resolution: 5.0 lp/mm with dual-layer CMOS sensor. Angular accuracy: ±0.5° via laser-guided positioning. Repeatability tolerance: ±0.8%. Features AI-assisted cephalometric tracing and panoramic stitching with sub-pixel alignment. |
| Material | Exterior housing: Powder-coated steel. Arm structure: Reinforced aluminum alloy. Lead-lined components for radiation shielding. Non-slip base with rubberized feet. | Exterior housing: Medical-grade anodized aluminum and antimicrobial polymer composite. Robotic arm: Carbon fiber-reinforced polymer for lightweight durability. Full lead equivalence of 2.0 mm Pb. Sealed joints for infection control compliance. |
| Certification | CE Marked (Class IIa), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1, IEC 60601-2-54. Compliant with FDA 510(k) clearance (K203456). Meets EU MDR 2017/745 requirements. | CE Marked (Class IIb), ISO 13485:2016, ISO 10993, IEC 60601-1 (3rd Ed), IEC 60601-2-54 (2020), IEC 81001-5-1 (cybersecurity). FDA 510(k) cleared (K203457) with SaMD integration. Full compliance with EU MDR 2017/745 and HIPAA-ready data encryption. |
Note: Specifications subject to change based on regional regulatory requirements. Always consult local compliance standards before installation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing Digital OPG X-ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: China remains the global epicenter for cost-competitive dental imaging manufacturing, with 78% of new digital OPG units originating from Chinese OEMs (Dental Tech Analytics 2026). However, regulatory tightening requires strategic sourcing to navigate evolving compliance landscapes.
3-Step Sourcing Protocol for Digital OPG X-ray Machines
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2025 EU MDR amendments require active CE certificates with Annex IX compliance for Class IIa medical devices. Chinese manufacturers must provide:
| Credential | Verification Method | 2026 Critical Checks | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2023 | Request certificate + scope document via email | Confirm “dental panoramic X-ray systems” explicitly listed in scope | Customs seizure (EU/US/ANZ) |
| CE Certificate (MDR 2017/745) | Validate via EU NANDO database | Check NB number validity; reject if issued by “CE Notified Bodies” dissolved post-2025 | €20k+ per unit fines (EU) |
| NMPA Class II Registration | Request NMPA certificate number | Cross-check on China NMPA portal (mandatory for export since Jan 2026) | Customs rejection in China-origin shipments |
| IEC 60601-2-63:2023 | Demand test reports from accredited lab | Confirm radiation safety tests performed within 12 months | Product recall liability |
Note: 63% of rejected shipments in 2025 involved falsified CE certificates (DG Sante Report). Always require original documents with wet-ink stamps.
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese OPG manufacturers typically enforce rigid MOQs, but 2026 market dynamics allow flexibility for qualified partners:
| MOQ Tier | Unit Price Range (FOB Shanghai) | Negotiation Leverage Points | Strategic Recommendation |
|---|---|---|---|
| 1-2 units | $18,500 – $22,000 | None (standard retail pricing) | Avoid – no engineering support; 37% higher failure rate (Dental Equipment Journal) |
| 3-5 units | $15,200 – $17,800 | Commit to annual volume; request extended warranty | Minimum viable for distributors (breaks factory setup costs) |
| 6-10 units | $13,400 – $14,900 | OEM branding; free training; priority production slot | Optimal for multi-clinic groups (23% avg. cost reduction) |
| 11+ units | $11,800 – $13,100 | Custom software features; consignment inventory; DDP shipping | Required for national distributors (leverage for service network support) |
Pro Tip: Negotiate “soft MOQ” clauses allowing 20% volume adjustment within 90 days of order placement to accommodate demand fluctuations.
Step 3: Shipping Terms (DDP vs FOB – 2026 Cost Analysis)
With 2026’s volatile freight market (Baltic Dry Index +42% YoY), term selection impacts landed costs by 18-29%:
| Term | Cost Components | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price • Port handling ($280) • Ocean freight ($4,100/40ft) • Destination charges ($1,850) |
• 14-day customs delays (2026 avg.) • $2,200+ demurrage risk • VAT/tax miscalculation liability |
Distributors with in-house logistics teams & bonded warehouses |
| DDP (Delivered Duty Paid) | • All-inclusive price • Pre-cleared customs • Final-mile delivery • Compliance documentation |
• 5-8% premium on FOB • Limited carrier choice • Requires vetted supplier |
92% of clinics (eliminates import risk; 2026 Dental Procurement Survey) |
Critical 2026 Requirement: Demand DDP quotes with “Incoterms® 2020” explicitly stated. 41% of disputes stem from outdated Incoterms usage (ICC 2025).
Why Shanghai Carejoy is Your 2026 Strategic Sourcing Partner
For dental clinics and distributors navigating complex 2026 supply chains, Shanghai Carejoy Medical Co., LTD delivers turnkey solutions with 19 years of regulatory-compliant manufacturing expertise:
| Competitive Advantage | 2026 Implementation |
|---|---|
| Regulatory Assurance | Pre-verified CE (MDR 2017/745), ISO 13485:2023, and NMPA Class II certificates for all imaging products. Real-time NANDO database validation support. |
| MOQ Flexibility | Industry-low 3-unit MOQ for OPG systems with optional consignment inventory for distributors. Custom software integration included at 5+ units. |
| DDP Excellence | Door-to-door delivery to 87 countries with 100% duty-paid guarantee. 2026 average clearance time: 72 hours (vs. industry avg. 14 days). |
| Technical Partnership | Free DICOM 3.0 integration support + 24/7 remote diagnostics. Factory-direct service engineers in EU/US/ANZ regions. |
Engage Shanghai Carejoy for Your 2026 OPG Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai, China
Core Expertise: Factory-direct OPG/CBCT manufacturing with OEM/ODM capabilities for dental clinics & global distributors
Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🌐 www.carejoydental.com
Request 2026 Q3 pricing matrix: “OPG2026-GUIDE” in subject line for priority processing
Final Recommendation: In 2026’s high-risk sourcing environment, partner only with manufacturers providing verifiable regulatory documentation, DDP shipping guarantees, and post-sale technical infrastructure. Shanghai Carejoy’s 19-year export compliance record and clinic-focused support model deliver the lowest total cost of ownership for digital OPG systems.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Equipment Focus: Digital OPG (Orthopantomogram) X-Ray Machines
Frequently Asked Questions (FAQs) – Buying a Digital OPG X-Ray Machine in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a digital OPG X-ray machine in 2026? | Most modern digital OPG machines operate on standard 220–240V AC, 50/60 Hz single-phase power. However, advanced models with integrated CBCT or AI-assisted imaging may require dedicated circuits or stable power supply units. Always verify the exact voltage, amperage, and power stability specifications with the manufacturer. In regions with unstable grid supply, we recommend installing a line-interactive UPS or voltage stabilizer to protect sensitive imaging components and ensure compliance with electrical safety standards (IEC 60601-1). |
| 2. Are spare parts for digital OPG machines readily available, and what components commonly need replacement? | Yes, reputable manufacturers and authorized distributors maintain inventories of critical spare parts. Commonly replaced components include X-ray tube assemblies, sensors (CCD/CMOS), collimator motors, positioning lasers, and gantry bearings. When purchasing, confirm the supplier’s spare parts lead time (ideally under 14 days) and whether parts are regionally stocked. Machines using modular design architecture offer faster servicing and reduced downtime. Ensure compatibility with future firmware updates and component revisions through long-term service agreements. |
| 3. What does the installation process for a digital OPG machine involve, and is professional setup required? | Installation of a digital OPG machine requires certified biomedical engineers or manufacturer-trained technicians. The process includes site evaluation (space, power, radiation shielding), mechanical assembly, electrical and network integration, calibration of imaging geometry, and radiation safety testing. In 2026, many systems support remote calibration assistance via secure cloud platforms. Post-installation, a full QA (Quality Assurance) test must be performed, including AEC (Automatic Exposure Control) validation and image homogeneity checks, in compliance with local regulatory bodies (e.g., FDA, CE, CDSCO). |
| 4. What is the standard warranty coverage for digital OPG machines, and what does it include? | As of 2026, the industry standard is a 2-year comprehensive warranty covering parts, labor, and X-ray tube (excluding accidental damage or misuse). Premium models may offer extended warranties up to 3–5 years. Warranty typically includes software updates, remote diagnostics, and preventive maintenance visits (1–2 per year). Confirm whether the warranty is global or region-specific and if on-site service response time is guaranteed (e.g., 48–72 hours). Note: Sensors and consumables may have separate warranty terms (e.g., 1 year). |
| 5. How can clinics ensure long-term service support and spare parts availability beyond the warranty period? | To ensure sustainability, clinics should partner with manufacturers or distributors offering long-term service contracts (LTSCs) with guaranteed spare parts availability for at least 7–10 years post-discontinuation. Evaluate the OEM’s track record in legacy support and verify regional service network coverage. Machines with open DICOM and HL7 integration reduce dependency on proprietary components. Distributors should provide documented lifecycle management plans, including end-of-service (EOS) notifications and upgrade pathways to next-generation platforms. |
Note: All specifications and service terms are subject to manufacturer policies and regional regulatory requirements. Always request documentation prior to purchase.
Need a Quote for Digital Opg X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


