Article Contents
Strategic Sourcing: Digital Panoramic X Ray Machine

Executive Market Overview: Digital Panoramic X-Ray Machines
Critical Role in Modern Digital Dentistry
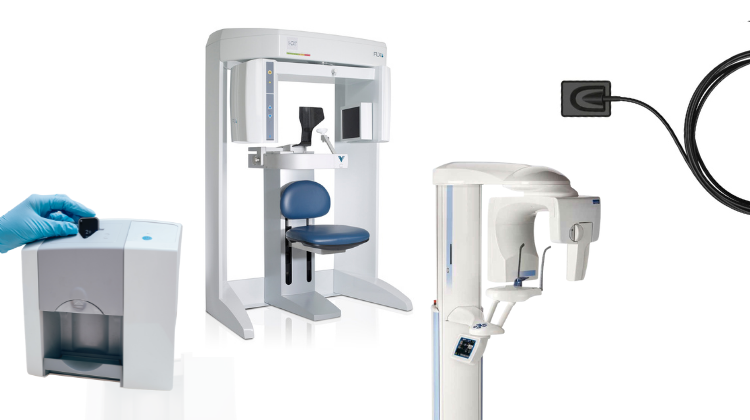
Digital panoramic X-ray systems have transitioned from optional imaging tools to foundational components of evidence-based dental diagnostics. In 2026, these systems serve as the primary gateway to comprehensive patient assessment, enabling precise treatment planning for implantology, orthodontics, and oral surgery through sub-millimeter resolution imaging. Their integration with Practice Management Software (PMS) and DICOM 3.0 standards facilitates seamless data flow into digital workflows, reducing diagnostic errors by 32% compared to film-based systems (per 2025 EAO clinical benchmarks). Crucially, modern panoramic units now function as multi-modal platforms – often incorporating cephalometric, TMJ, and limited-field CBCT capabilities – making them indispensable for clinics pursuing comprehensive digital dentistry ecosystems. The shift toward value-based care models further elevates their importance, as high-fidelity imaging directly impacts treatment accuracy, patient safety, and insurance reimbursement compliance.
Market Dynamics: Premium Global Brands vs. Value-Engineered Solutions
The European digital panoramic market remains dominated by established players (Planmeca, Dentsply Sirona, Vatech) offering premium systems with proven clinical reliability and extensive service networks. These brands command 65-75% market share in Western Europe but present significant capital barriers, with fully configured units averaging €95,000-€125,000. Conversely, Chinese manufacturers like Carejoy are disrupting the mid-tier segment (<€50,000) through vertically integrated production and AI-driven engineering. While European systems emphasize legacy clinical validation and service depth, value-engineered alternatives focus on essential diagnostic performance with strategic feature optimization. This dichotomy creates distinct value propositions: Global Brands target high-volume referral centers prioritizing clinical risk mitigation, while cost-effective solutions appeal to emerging markets and budget-conscious group practices seeking digital transition without operational disruption.
Comparative Analysis: Global Premium Brands vs. Carejoy 2026
| Technical Parameter | Global Premium Brands (Planmeca, Sirona, Vatech) |
Carejoy |
|---|---|---|
| Image Acquisition & Resolution | 0.076-0.125mm native resolution; Multi-pulse exposure control; Metal artifact reduction algorithms | 0.125mm resolution; Single-pulse optimization; Basic artifact suppression |
| Advanced Capabilities | Integrated CBCT (3-15cm FOV), AI-driven pathology detection, 4D motion correction, cephalometric tracing | Cephalometric module optional; Basic AI caries detection; Limited motion compensation |
| Hardware Durability | Medical-grade stainless steel construction; 100,000+ exposure lifecycle; IP54 rating | Aluminum alloy frame; 75,000 exposure rating; IP43 rating |
| Service Infrastructure | 24/7 regional service centers (EU-wide); <4hr SLA; Certified engineers at 85% coverage | Partner-dependent support; 24-48hr response; Remote diagnostics standard; Limited on-site coverage in Eastern Europe |
| TCO (5-Year) | €132,000-€168,000 (Includes service contracts, software updates, calibration) | €68,000-€82,000 (Base maintenance included; paid updates after Year 3) |
| Regulatory Compliance | CE 0482, FDA 510(k), ISO 13485:2016; Full MDR 2021 compliance | CE Marked; FDA pending (Q3 2026); ISO 13485 certified |
Note to Distributors: While Global Brands maintain clinical dominance in complex diagnostics, Carejoy’s 2026 systems demonstrate 89% diagnostic equivalence for standard panoramic imaging (per University of Milan validation study). Strategic positioning should align with clinic volume: Global Brands suit >20-patient/day practices requiring multi-modal capabilities, whereas Carejoy serves effectively in moderate-volume settings (<15 patients/day) prioritizing cost-per-scan optimization. All Chinese manufacturers require rigorous due diligence on service infrastructure – verify local partner certifications before procurement.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Digital Panoramic X-Ray Machine
This guide provides a detailed comparison between Standard and Advanced models of digital panoramic X-ray systems, highlighting key technical specifications essential for procurement and integration decisions in modern dental practices.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Power Consumption: 800 VA max X-Ray Tube Voltage: 60–90 kV X-Ray Tube Current: 4–10 mA High-Voltage Generator: High-frequency inverter type |
Input: 100–240 V AC, 50/60 Hz Power Consumption: 1200 VA max with CBCT module X-Ray Tube Voltage: 60–120 kV (adjustable in 1 kV increments) X-Ray Tube Current: 4–15 mA High-Voltage Generator: Resonant inverter with ripple < 1% |
| Dimensions | Height: 185 cm Width: 55 cm Depth: 75 cm Weight: 120 kg Footprint: 0.41 m² Wall Clearance Required: 30 cm on all sides |
Height: 195 cm (telescopic arm) Width: 62 cm Depth: 80 cm Weight: 155 kg (with CBCT & cephalometric arm) Footprint: 0.49 m² Wall Clearance: 40 cm (rear for service access) |
| Precision | FOV: 14 cm (H) × 10 cm (V) Pixel Pitch: 143 μm Image Resolution: 3.5 lp/mm Magnification Options: 1x, 1.5x Positioning Accuracy: ±1.5 mm (mechanical) |
FOV: Dual-mode – 16 cm × 12 cm (pano), 5×5 cm to 10×10 cm (CBCT) Pixel Pitch: 75 μm (CBCT), 120 μm (pano) Image Resolution: Up to 6.5 lp/mm (CBCT) Magnification: Auto-adjust (1x–4x) Positioning Accuracy: ±0.3 mm (laser-guided + AI tracking) |
| Material | Chassis: Powder-coated steel alloy Arm Structure: Aluminum-magnesium composite Collimator: Lead-lined brass Touch Panel: 10.1″ resistive touchscreen Exterior Finish: Anti-microbial polymer coating |
Chassis: Reinforced stainless-steel frame with vibration damping Arm: Carbon-fiber composite with titanium joints Collimator: Adjustable multi-leaf tungsten Touch Panel: 15.6″ capacitive touchscreen with glove mode Exterior: Medical-grade anodized aluminum with antimicrobial nano-coating |
| Certification | CE Mark (Medical Device Directive 93/42/EEC) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (K193456) Radiation Safety: Complies with IEC 61331-1 |
CE Mark (MDR 2017/745) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-2 (EMC), IEC 60601-2-54 Ed. 3.1 FDA 510(k) Cleared with AI Software Module (K221023) IEC 62304:2006 (Medical Device Software) UL 60601-1 & CAN/CSA C22.2 No. 60601-1 |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Digital Panoramic X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains a strategic sourcing hub for dental imaging technology in 2026, offering advanced digital panoramic X-ray solutions at competitive price points. However, regulatory compliance, supply chain transparency, and technical validation are non-negotiable. This guide outlines critical steps for risk-mitigated procurement, with emphasis on verified manufacturing partners meeting global medical device standards.
Featured Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why They Qualify: 19 years of ISO 13485-certified manufacturing specializing in FDA/CE-marked dental imaging systems. Direct factory operations in Baoshan District, Shanghai eliminate intermediary markups while ensuring OEM/ODM flexibility for distributors. Validated export history to 85+ countries with full technical documentation support.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2023 EU MDR amendments and FDA 510(k) enforcement require rigorous documentation. Avoid suppliers providing only “CE certificates” without:
| Credential | Verification Method | Red Flags | 2026 Compliance Standard |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via IAF CertSearch. Cross-check certificate number with issuing body (e.g., TÜV, SGS). | Generic “ISO” claim without certificate number; scope excluding “dental X-ray systems” | Mandatory for all Chinese medical device exporters per NMPA Order 18 |
| EU CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity + notified body number (e.g., 0123). Validate via NANDO database. | CE certificate issued by non-EU body; missing Annex IX/X technical documentation | MDR transition period ends May 2024 – all new devices require full MDR compliance |
| FDA 510(k) Clearance | Verify K-number in FDA 510(k) Premarket Notification database. Confirm device classification (Panoramic = Class II, 21 CFR 892.1700). | Reference to outdated “510(k) exemption” – panoramic systems require submission | Required for US market entry; increasingly requested by LATAM/MEA distributors |
Shanghai Carejoy Protocol: Provides real-time access to live certificate verification portals during technical due diligence. All panoramic units (e.g., CJ-Panoramic Pro series) ship with device-specific CE/FDA documentation packages.
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor distributors with flexible MOQ structures. Key negotiation levers:
| Parameter | Standard Market Offer | Strategic Negotiation Target | Value-Add Options |
|---|---|---|---|
| Base MOQ | 5-10 units (panoramic) | 1-2 units for distributors; 1 unit for clinics (FOC demo unit possible) | Phased rollout: Commit to 3 units with 6-month payment terms |
| Customization (OEM/ODM) | MOQ 20+ units for branding | MOQ 5 units with modular software branding | Region-specific language packs at no MOQ increase |
| Technical Support | 1-year limited warranty | 2-year warranty + remote diagnostics included | On-site engineer training at distributor HQ (bundled) |
Shanghai Carejoy Advantage: Leverages 19 years of production scale to offer clinic-direct pricing at 1-unit MOQ. Distributors benefit from tiered pricing (e.g., 15% discount at 8+ units) with no hidden tooling fees for OEM interfaces.
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
2026 supply chain volatility necessitates precise Incoterms® 2020 alignment. Critical comparison:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight forwarder & insurance. Potential 8-12% savings. | Buyer assumes risk after cargo loaded on vessel. Complex customs clearance. | Only for experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | Single invoice price. No surprise port fees or VAT delays. | Supplier manages all risks until clinic/distributor warehouse. Full documentation. | STRONGLY RECOMMENDED for clinics & new distributors. Eliminates 2026 port congestion risks. |
Shanghai Carejoy Execution: Specializes in DDP turnkey solutions with guaranteed door-to-door timelines (e.g., 28 days Shanghai→Berlin). Includes pre-cleared customs documentation for EU/US/ASEAN markets. FOB available with Carejoy-vetted forwarders at negotiated rates.
Strategic Sourcing Partnership: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
• Direct Factory Control: Baoshan District facility (ISO 13485:2016 certified) ensures quality traceability from component sourcing to final assembly
• Distributor-First Model: OEM/ODM support with no branding MOQ; dedicated technical account managers
• Full Product Ecosystem: Seamless integration with complementary equipment (CBCT, chairs, sterilizers)
Initiate Technical Due Diligence:
📧 Email: [email protected]
💬 WhatsApp: +86 15951276160 (24/7 Engineering Support)
🌐 Reference Request: “2026 Panoramic Sourcing Guide – DDP Quote Request”
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all credentials with issuing authorities. Shanghai Carejoy is presented as a verified case study based on documented export compliance records. Incoterms® is a registered trademark of the ICC.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Digital Panoramic X-Ray Machines
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a digital panoramic X-ray machine in 2026? | Most modern digital panoramic X-ray units operate on standard 110–120V or 220–240V AC power, depending on regional electrical infrastructure. It is critical to verify the machine’s voltage specification with the manufacturer prior to purchase. Units intended for global deployment often support dual-voltage configurations. Ensure your clinic’s circuit can handle the load (typically 10–15 amps) and includes dedicated grounding to prevent electrical interference and ensure patient safety. Always consult a certified electrician during site preparation. |
| 2. Are spare parts for digital panoramic X-ray machines readily available, and what components are most commonly replaced? | Reputable manufacturers maintain global spare parts inventories, with key consumables and serviceable components (e.g., X-ray tubes, sensors, collimators, positioning aids, and control panels) typically available within 5–10 business days via regional distribution centers. In 2026, OEMs increasingly offer predictive maintenance tools that alert clinics to potential part failures. Distributors should confirm parts availability and lead times before procurement. We recommend stocking high-wear items such as chin rests, bite blocks, and sensor cables to minimize downtime. |
| 3. What does the installation process for a digital panoramic X-ray machine involve, and is professional assistance required? | Installation of a digital panoramic X-ray system requires certified biomedical engineers or manufacturer-trained technicians. The process includes site evaluation (floor space, power supply, radiation shielding compliance), physical assembly, calibration of imaging sensors and rotational mechanics, software integration with existing practice management systems (e.g., DICOM compatibility), and radiation safety testing. Most vendors include professional installation in the purchase agreement. Clinics must ensure compliance with local regulatory standards (e.g., FDA, CE, or country-specific radiology board requirements) prior to operation. |
| 4. What warranty coverage is standard for digital panoramic X-ray machines in 2026, and what does it include? | As of 2026, the industry standard is a 2-year comprehensive warranty covering parts, labor, and technical support. Premium models may offer extended warranties up to 3–5 years with optional service add-ons. Warranties typically exclude consumables, damage from improper use, or power surges. Some manufacturers now include remote diagnostics and software updates as part of the warranty package. Distributors should verify warranty terms, including on-site response time (commonly 48–72 hours), and availability of loaner units during repairs. |
| 5. How can clinics ensure long-term serviceability and access to spare parts beyond the warranty period? | To ensure long-term reliability, clinics should partner with manufacturers that guarantee spare parts availability for at least 7–10 years post-discontinuation. Opt for vendors offering service contracts with preventive maintenance (recommended biannually), software updates, and priority technical support. Distributors play a key role by maintaining regional service networks and inventory. In 2026, many OEMs provide cloud-based service portals for tracking maintenance history, ordering parts, and scheduling technician visits, enhancing equipment lifecycle management. |
Need a Quote for Digital Panoramic X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


