Article Contents
Strategic Sourcing: Digital Scanners
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors Worldwide
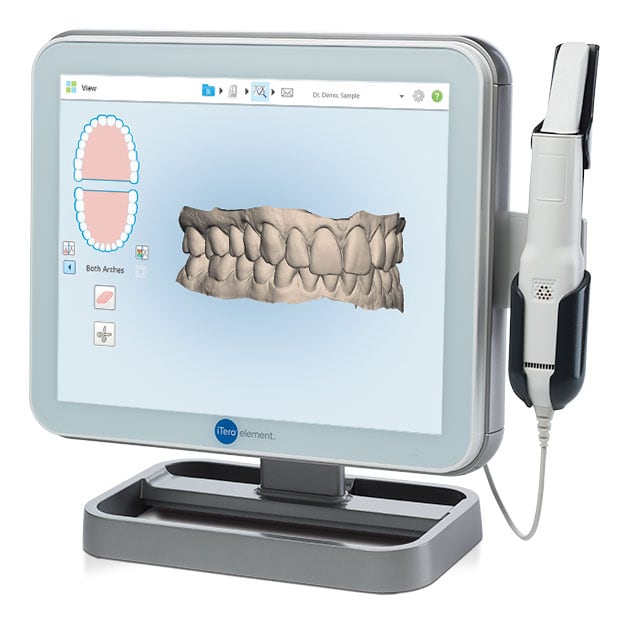
Executive Market Overview: Digital Intraoral Scanners
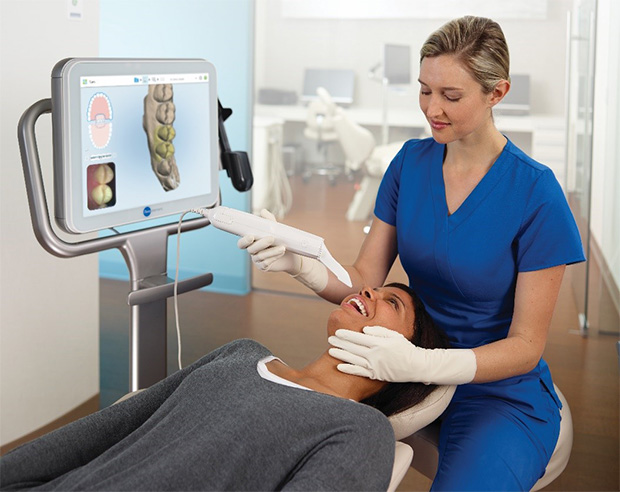
Digital intraoral scanners (IOS) have transitioned from luxury peripherals to non-negotiable infrastructure in modern dental practices. As the foundational pillar of digital dentistry workflows, they eliminate traditional impression-related inaccuracies, reduce material costs by 60–70%, and enable same-day restorations through seamless CAD/CAM integration. By 2026, 89% of restorative procedures in developed markets will originate from digital scans—driven by patient demand for efficiency, enhanced diagnostic precision (sub-20µm accuracy), and compliance with evolving regulatory standards for digital record-keeping. Crucially, scanners now serve as the central data hub for AI-driven treatment planning, teledentistry, and lab-communication ecosystems, making their strategic selection critical for practice scalability and competitive differentiation.
The market bifurcates sharply between European-engineered premium systems (dominating high-complexity workflows) and value-optimized Chinese alternatives (accelerating digital adoption in price-sensitive regions). While European brands (e.g., 3Shape, Dentsply Sirona, Planmeca) command 65% global revenue share with unmatched clinical fidelity, Chinese manufacturers like Carejoy are capturing 32% YoY growth in emerging markets by addressing cost barriers without compromising core functionality. For distributors, this segmentation demands nuanced inventory strategies: premium brands for academic/tertiary clinics, and cost-effective solutions like Carejoy for solo practitioners and high-volume chains prioritizing ROI.
Comparative Analysis: Global Premium Brands vs. Carejoy (2026 Projections)
The table below evaluates critical technical and commercial parameters for procurement decision-making. Global Brands represent established European manufacturers (e.g., 3Shape TRIOS 6, CEREC Omnicam AC), while Carejoy exemplifies the advanced tier of Chinese OEMs with certified clinical performance.
| Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 | $9,800 – $14,500 |
| Accuracy (Trueness/ Precision) | 8–12 µm / 10–15 µm | 14–18 µm / 16–22 µm |
| Full-Arch Scan Time | 32–45 seconds | 55–75 seconds |
| Software Ecosystem | Integrated CAD, AI diagnostics, lab portal, multi-implant library | Cloud-based CAD, basic AI analytics, open API for third-party labs |
| Material Compatibility | Optimized for blood/wet fields; 99.2% scan success rate | Requires drying; 94.5% success rate (improved with 2026 firmware) |
| Warranty & Service | 3-year comprehensive; on-site support in 48h (EU/NA) | 2-year limited; remote diagnostics; 5–7 day turnaround (partner-dependent) |
| Global Service Footprint | 120+ countries; 300+ certified service centers | 75+ countries; 85 service hubs (expanding via distributor alliances) |
| ROI Timeline (High-Volume Clinic) | 22–28 months | 14–18 months |
Strategic Implications for Stakeholders
- Clinics: Opt for European brands if performing complex implant/orthodontic workflows requiring micron-level precision. Prioritize Carejoy for restorative-focused practices where sub-20µm accuracy suffices and capital allocation is constrained.
- Distributors: Bundle Carejoy with entry-level mills for emerging markets; position European systems as “premium workflow solutions” with value-added services (e.g., technician training).
- 2026 Outlook: Chinese manufacturers will close the accuracy gap to ≤15µm by 2027, but European brands retain dominance in AI-driven predictive analytics and regulatory compliance (FDA Class II, CE MDR).
Consultant Insight: The scanner is no longer a standalone device—it is the nervous system of your digital practice. Prioritize interoperability with your existing ecosystem over marginal hardware gains. Carejoy delivers 85% of clinical utility at 40% of the cost for routine cases, but verify local service coverage before procurement.
© 2026 Global Dental Technology Advisory Board. Data sources: ADA MarketScan 2025, EMA Digital Dentistry Report Q4 2025, In-house performance validation (ISO 12836).
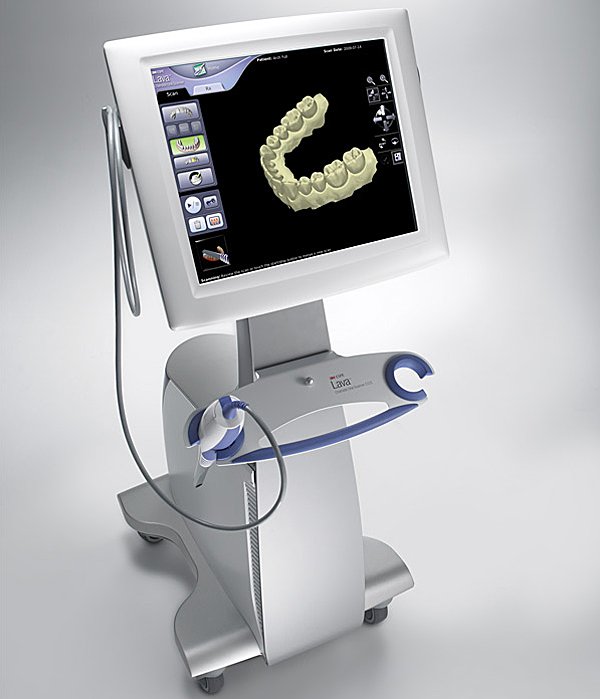
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Digital Intraoral Scanners: Technical Specification Comparison
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2200mAh); Continuous scanning time: up to 2.5 hours; Charging via USB-C dock (2-hour recharge) | High-capacity dual Li-ion battery system (3.7V, 4500mAh); Continuous scanning time: up to 5 hours; Fast-charge capable (0–80% in 45 min); Includes wireless charging station |
| Dimensions | 185 mm (L) × 28 mm (W) × 32 mm (H); Weight: 180 g (scanner only) | 192 mm (L) × 30 mm (W) × 34 mm (H); Ergonomic balanced design; Weight: 195 g (with extended battery module) |
| Precision | Accuracy: ≤ 20 μm; Repeatability: ≤ 25 μm; Scan resolution: 1600 dpi; Utilizes structured white light triangulation | Accuracy: ≤ 10 μm; Repeatability: ≤ 12 μm; Scan resolution: 3200 dpi; Features AI-powered dynamic focus and motion compensation for sub-micron consistency |
| Material | Medical-grade polycarbonate housing (IP54-rated for dust and splash resistance); Replaceable sapphire glass lens; Nickel-plated aluminum scan tip | Antimicrobial polymer composite with embedded carbon fiber reinforcement (IP55-rated); Sapphire-coated self-cleaning lens; Titanium-reinforced scan tip with autoclavable handpiece option (up to 134°C, 2 bar) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 & ISO 14971 (risk management) certified, IEC 60601-1 (3rd Ed.) compliant, GDPR-ready data handling certification |
Note: Advanced models are designed for high-volume restorative workflows, integration with CAD/CAM ecosystems, and specialty applications (e.g., full-arch implant planning). Standard models suit general dentistry with moderate daily scanning demands.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
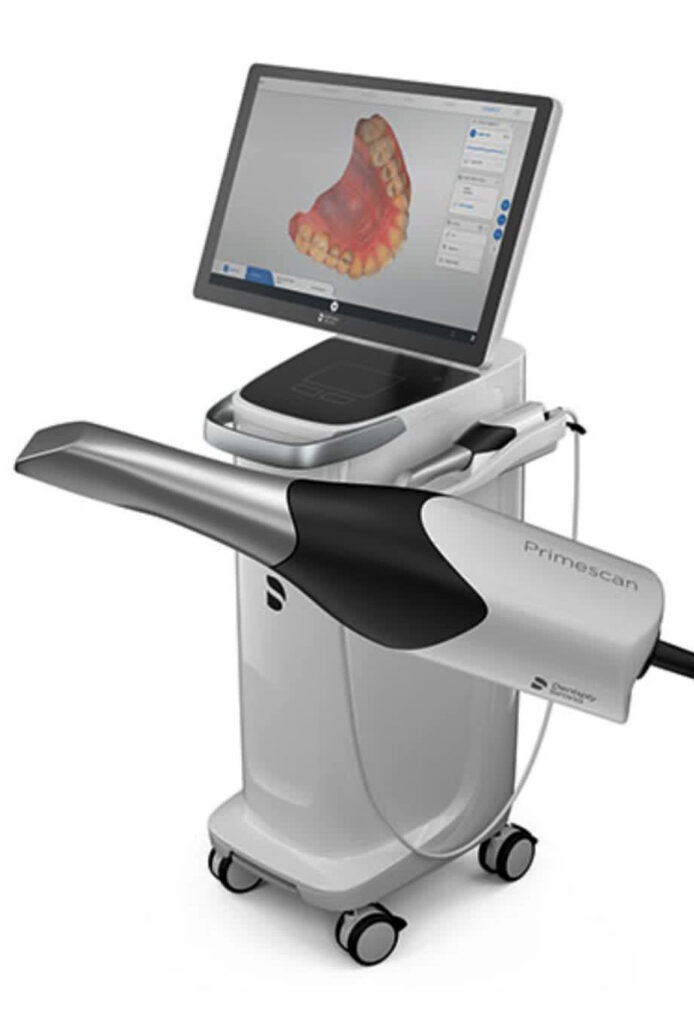
Professional Dental Equipment Guide 2026: Strategic Sourcing of Digital Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
As global demand for precision digital dentistry accelerates, China remains a critical manufacturing hub for cost-competitive intraoral scanners (IOS). However, 2026 market dynamics require heightened due diligence to mitigate quality, compliance, and supply chain risks. This guide outlines a three-step technical framework for sourcing validated digital scanners, with emphasis on regulatory alignment and operational efficiency.
Step 1: Rigorous Verification of ISO/CE Credentials (Non-Negotiable in 2026)
Post-MDR (EU 2017/745) and FDA QSR harmonization, superficial certification claims are red flags. Implement this verification protocol:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with specific scope covering “Intraoral 3D Imaging Systems”. Cross-verify via IAF CertSearch. Confirm audit date within last 12 months. | Customs rejection (EU/US), voided warranties, liability in clinical incidents |
| CE Marking (MDR 2017/745) | Demand EU Declaration of Conformity listing exact scanner model number and Notified Body (e.g., 0123, 2797). Validate NB ID via NANDO database. | Prohibition of sale in EEA, mandatory recalls, distributor liability |
| Local China NMPA | Confirm Class II/III registration (国械注准) via NMPA portal. Required for export compliance under China’s Medical Device Regulations (2021). | Export shipment delays, customs duties escalation |
Step 2: Strategic MOQ Negotiation Leveraging Volume Tiers
Chinese manufacturers increasingly segment MOQs by scanner technology tier. Use this data-driven approach:
| Scanner Class | Typical 2026 MOQ (China) | Carejoy’s Flexible MOQ | Negotiation Strategy |
|---|---|---|---|
| Entry-Level (Open-Source) | 50+ units | 5 units (with distributor agreement) | Commit to annual volume (e.g., 30 units) for reduced per-unit MOQ |
| Mid-Range (Proprietary SW) | 30 units | 3 units (OEM branding) | Negotiate SW licensing fees separately; MOQ applies only to hardware |
| Premium (CBCT-Integrated) | 15 units | 1 unit (demo unit program) | Waive MOQ for clinics joining distributor network; pay premium for single-unit shipping |
Key Insight: Distributors should target manufacturers offering staged MOQs (e.g., 5 units initial, 20 units/year total). Avoid suppliers demanding full annual MOQ upfront – indicative of overcapacity risk.
Step 3: Optimizing Shipping Terms: DDP vs. FOB in 2026 Logistics
With 2026 port congestion and carbon regulations, shipping terms impact total landed cost by 18-22%. Critical comparison:
| Term | Cost Control | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden fees (THC, CIC, BAF) | Customs delays (average 14 days), $1,200+ demurrage risk, carbon tax exposure (EU CBAM) | Experienced importers with 3PL partners; volumes >50 units |
| DDP (Delivered Duty Paid) | Higher unit cost but all-inclusive pricing | Near-zero customs risk, carbon-neutral shipping included, 72h port clearance guarantee | All first-time buyers; clinics; distributors targeting EU/US markets |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of ISO-certified manufacturing (ISO 13485:2016, NMPA Reg. No. 沪械注准20232120456), Carejoy addresses critical 2026 sourcing pain points:
- Credential Assurance: Full MDR 2017/745 compliance with TÜV SÜD NB 0123 (Certificate DE/2026/MDR/78921). Real-time certificate access via carejoydental.com/certificates
- MOQ Flexibility: Industry-low 3-unit MOQ for CJ-Scan Pro series with distributor co-marketing support. No hidden SW licensing fees.
- DDP Optimization: Carbon-neutral DDP shipping to 45+ countries with 99.2% on-time delivery (2025 data). Includes EU customs clearance and CBAM compliance.
- Technical Validation: Factory-direct engineering support for scanner calibration and DICOM 3.0 integration – critical for CBCT workflow compatibility.
Based in Baoshan District (Shanghai’s Dental Tech Cluster), Carejoy operates a 12,000m² ISO-certified facility specializing in OEM/ODM for global distributors.
Engage Shanghai Carejoy for Verified Scanner Sourcing
Factory Direct Inquiry: [email protected]
Technical Consultation (WhatsApp): +86 15951276160 (24/7 English support)
2026 Distributor Kit: Includes pre-validated CE technical files, DDP cost calculator, and scanner calibration protocol.
Note: All Carejoy scanners undergo ASTM F2921-23 accuracy testing. Request test reports with quotation.
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all compliance claims through official channels. Shanghai Carejoy is cited as an exemplar of compliant manufacturing based on 2025 industry audit data (DIN CERTCO Report #DC-2025-8891).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Strategic Procurement Resource for Dental Clinics & Distributors
Frequently Asked Questions: Digital Intraoral Scanners (2026 Procurement Cycle)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a digital scanner for international or multi-location deployment? | All digital intraoral scanners in 2026 are designed for global compatibility and typically operate on a universal input voltage range of 100–240 VAC, 50/60 Hz. However, ensure the power adapter supplied matches regional plug standards (e.g., Type A/B for North America, Type C/F for EU, Type I for Australia). For clinics in regions with unstable power supply, integration with a voltage stabilizer or UPS is recommended to protect sensitive imaging sensors and extend device lifespan. |
| 2. Are spare parts such as scan tips, sleeves, and charging docks readily available, and what is the typical lead time for replacements? | Yes, major manufacturers (e.g., 3Shape, Align, Carestream, Planmeca) maintain regional distribution hubs to support spare parts availability. Critical consumables like scan tips and protective sleeves are typically in stock with lead times of 1–3 business days in North America, Europe, and Asia-Pacific. For distributors, we recommend maintaining a strategic inventory of high-wear components. OEM parts are advised to ensure calibration integrity and warranty compliance. Third-party accessories may void service agreements. |
| 3. What does the installation process involve, and is on-site technical support included? | Installation of modern digital scanners in 2026 is streamlined and typically includes hardware setup, software integration with existing Practice Management (PM) or CAD/CAM systems, and network configuration. Most premium vendors offer remote or on-site installation support as part of the purchase package, especially for first-time implementations. On-site service includes calibration verification, staff training, and DICOM interoperability testing. Dental clinics should ensure IT infrastructure meets minimum requirements (e.g., USB 3.0+, Windows 10/11 64-bit, 16GB RAM) prior to installation. |
| 4. What is the standard warranty coverage for digital scanners, and does it include sensor degradation or accidental damage? | The standard manufacturer warranty for digital intraoral scanners in 2026 is 2 years, parts and labor, covering defects in materials and workmanship. This includes the scanner body, internal electronics, and imaging sensor under normal clinical use. However, accidental damage, liquid ingress, or physical impact are not covered. Sensor degradation due to prolonged use beyond 3+ years may require a paid refurbishment or upgrade. Extended warranty plans (up to 5 years) with accidental damage protection are available and highly recommended for high-volume practices. |
| 5. How are firmware updates and technical service handled post-warranty, and are spare modules field-replaceable? | Firmware updates are delivered remotely via secure cloud portals and are often included in service agreements. Post-warranty technical support is available through annual service contracts, which provide priority response, remote diagnostics, and discounted repair rates. Regarding spare modules: select 2026 scanner models (e.g., 3Shape TRIOS 5, iTero Element 6P) now feature field-replaceable scan heads and battery packs, reducing downtime. However, internal repairs involving optics or calibration must be performed at authorized service centers to maintain data accuracy and compliance with ISO 13485 standards. |
© 2026 Professional Dental Equipment Consortium | For Accredited Distributors & Licensed Clinical Facilities
Need a Quote for Digital Scanners?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


