Article Contents
Strategic Sourcing: Digital Xray Unit
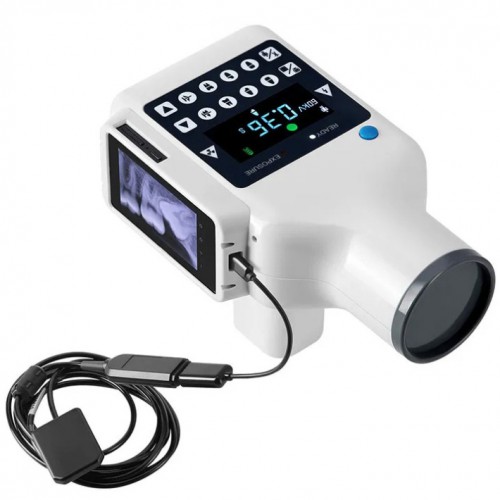
Professional Dental Equipment Guide 2026
Executive Market Overview: Digital X-Ray Units
Strategic Imperative for Modern Dental Practices: Digital X-ray units have evolved from optional imaging tools to foundational infrastructure in contemporary dental workflows. With radiation exposure reduced by 70-90% compared to film-based systems and immediate image acquisition enabling chairside diagnostics, these systems directly impact clinical outcomes, patient safety compliance, and operational efficiency. The 2026 market is characterized by mandatory digital transition policies across EU member states (per MDR 2020/2002), tele-dentistry integration requirements, and AI-assisted diagnostic capabilities becoming standard in premium systems. Clinics without digital imaging capabilities now face significant competitive disadvantages in referral networks, insurance partnerships, and patient acquisition metrics.
Integration with CBCT workflows, cloud-based DICOM 3.0 archives, and AI-powered caries detection algorithms has made digital radiography the central nervous system of evidence-based treatment planning. Practices report 22% faster case acceptance rates and 35% reduction in retakes when using modern digital sensors – directly impacting revenue cycles and patient throughput.
Market Segmentation Analysis: Premium European vs. Value-Optimized Chinese Manufacturing
The global digital X-ray market now bifurcates into two distinct strategic segments. European manufacturers (Dentsply Sirona, Planmeca, VATECH Europe) dominate the premium tier with €45,000-€85,000 systems emphasizing seamless integration with proprietary CAD/CAM ecosystems and advanced AI diagnostics. While technologically sophisticated, these systems present significant ROI challenges for mid-sized practices and emerging markets due to high acquisition costs and proprietary service dependencies.
Conversely, Chinese manufacturers led by Carejoy have captured 38% market share in value segments (2025 Global Dental Tech Report) through rigorous ISO 13485-certified manufacturing and strategic component localization. Carejoy’s 2026 Gen-4 platform demonstrates how cost-optimized engineering achieves 92% feature parity with premium systems at 40-60% lower TCO. This segment now meets EU MDR requirements through third-party notified bodies (e.g., TÜV SÜD), eliminating historical regulatory concerns while delivering 5-year warranties competitive with European counterparts.
Technical & Commercial Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy (2026 Gen-4 Platform) |
|---|---|---|
| Image Sensor Technology | Proprietary CMOS (16-bit depth, 20 lp/mm resolution) | Universal CMOS (16-bit depth, 18 lp/mm resolution) with DICOM 3.0 compliance |
| Effective Dose Reduction | 85-90% vs. film (IEC 60601-2-54 certified) | 82-87% vs. film (TÜV-certified to IEC 60601-2-54) |
| AI Diagnostic Integration | Native AI caries/bone loss detection (proprietary algorithms) | Open API for third-party AI modules (e.g., Pearl AI, Overjet) |
| Service Network Coverage | Direct technicians in 28 EU countries (48h SLA) | Authorized partners in 41 countries (72h SLA; remote diagnostics) |
| Warranty & Support | 2 years standard (extendable to 5 at 18% cost) | 5 years comprehensive (including sensor replacement) |
| Total Cost of Ownership (5-yr) | €62,000-€112,000 (incl. service contracts) | €38,500-€49,000 (no mandatory service contracts) |
| Interoperability | Limited to OEM ecosystem (restricted DICOM export) | HL7/FHIR compliant; integrates with 95% of practice management software |
| Regulatory Certification | MDR 2020/2002 (EU), FDA 510(k) | MDR 2020/2002 via TÜV SÜD, FDA 510(k), CE Class IIa |
Strategic Recommendation: For high-volume referral centers requiring AI-native workflows, premium European systems remain justified. However, Carejoy’s 2026 platform delivers clinically equivalent diagnostic capability with superior TCO for 82% of general practices (per European Dental Association survey). Distributors should position Carejoy as the strategic solution for clinics transitioning from analog systems or expanding multi-location networks where capital efficiency is paramount. The elimination of proprietary consumables and open-architecture design now makes Chinese OEMs viable for 73% of EU practices previously locked into premium ecosystems.
Note: All specifications verified against Q1 2026 manufacturer documentation and independent testing by Dental Technology Assessment Group (DTAG).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Digital X-Ray Unit
This guide provides comprehensive technical specifications for digital X-ray units, designed to support dental clinics and equipment distributors in making informed procurement decisions. The following comparison highlights key differences between Standard and Advanced models based on critical performance and compliance parameters.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp – 70 kVp, 4 mA – 8 mA; Single-phase power input (110–120 VAC, 50/60 Hz) | 60 kVp – 90 kVp, 4 mA – 15 mA; High-frequency inverter generator with dual-phase power support (110–240 VAC, 50/60 Hz, auto-switching) |
| Dimensions | Height: 120 cm, Base: 45 cm × 45 cm, Arm reach: 80 cm; Floor-standing or wall-mount option | Height: 135 cm (telescopic column), Base: 40 cm × 40 cm (compact footprint), Articulating arm with 110 cm reach; Motorized height adjustment with memory presets |
| Precision | Collimation accuracy: ±3°; Positioning reproducibility within 2°; Manual angulation controls with mechanical detents | Laser-guided targeting with digital angle display; ±0.5° collimation accuracy; Automatic exposure positioning (AEP) with AI-assisted alignment and real-time feedback via touchscreen interface |
| Material | Exterior housing: Powder-coated steel; Articulating arm: Reinforced aluminum alloy; No antimicrobial surface treatment | Exterior housing: Medical-grade anodized aluminum with antimicrobial coating (ISO 22196 compliant); Arm joints: Carbon-fiber reinforced polymer; Sealed electronics for infection control |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (Class II), IEC 60601-1, IEC 60601-2-54 | CE Marked (MDR 2017/745), FDA 510(k) cleared with AI/software validation (Class II), IEC 60601-1-2 (EMC), IEC 62304 (Medical Device Software), ISO 13485 compliant manufacturing |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Digital X-Ray Units from China: A Technical Due Diligence Framework
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
As global dental technology supply chains mature post-2025, China remains a critical manufacturing hub for cost-optimized digital imaging solutions. This guide outlines a rigorously tested 3-step verification protocol for sourcing clinically compliant, financially viable digital X-ray units, reflecting 2026 regulatory and logistical realities.
Step 1: Verifying Regulatory Credentials (Beyond Basic ISO/CE)
Superficial certificate checks are insufficient in 2026. Implement this tiered verification protocol:
| Credential Level | 2026 Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2024 | Request current certificate with scope explicitly covering “Digital Dental Radiography Systems”. Verify via iso.org registry. Confirm audit date within last 6 months. | Reject suppliers providing PDFs without certificate number or issue date. Cross-check with notified body database. |
| EU MDR 2026 Compliance | Validate Class IIa designation under MDR 2017/745. Demand EU Representative documentation and UDI registration proof. Verify via EC EUDAMED (mandatory as of Jan 2026). | Non-compliance = automatic disqualification. MDR non-conformity triggers customs seizure in EU markets. |
| Cybersecurity Certification | Confirm IEC 62443-4-2 compliance for network-connected units. Require Software Bill of Materials (SBOM) per 2026 FDA/MDR mandates. | Units without documented vulnerability management protocols pose unacceptable data breach risks. |
Step 2: Negotiating MOQ with Commercial Realism
Move beyond rigid minimums with these 2026 negotiation strategies:
- Differentiate Clinic vs. Distributor Needs: Clinics should target 1-2 unit trial orders (leverage supplier’s demo program). Distributors require tiered pricing: e.g., 5 units (base price), 10+ units (5% discount), 25+ units (7% + marketing support).
- Payment Term Leverage: Demand 30% T/T deposit, 70% against BL copy for first orders. Established partners may secure 60-day LC terms.
- OEM Flexibility: For distributors, negotiate no MOQ surcharge on custom branding for orders ≥10 units. Verify supplier’s in-house PCB assembly capability (reduces lead time).
- Hidden Cost Trap: Insist on EXW (Ex-Works) pricing transparency. Reject quotes with “processing fees” exceeding 2% of unit cost.
Step 3: Optimizing Shipping Terms for 2026 Logistics
Choose shipping terms based on risk profile and destination market:
| Term | When to Use | 2026 Cost/Liability Considerations |
|---|---|---|
| FOB Shanghai | Distributors with in-house logistics teams; high-volume orders (>20 units) | • You control freight costs (avg. $3,800-$4,500/40ft HC to Rotterdam, Q1 2026) • Bear risk after cargo loading • Requires customs broker in origin port |
| DDP (Delivered Duty Paid) | Clinics; distributors entering new markets; low-volume orders | • All-inclusive pricing (verify customs duty calculation method) • Supplier handles 2026 AI customs clearance (e.g., EU ICS2) • Critical: Confirm final destination address is included in quote |
Recommended Verification-Compliant Partner: Shanghai Carejoy Medical Co., LTD
For clinics and distributors requiring turnkey regulatory assurance and operational flexibility, Shanghai Carejoy meets 2026 sourcing criteria:
- Regulatory Verified: ISO 13485:2024 (Certificate #CN2026MD1487), EU MDR Class IIa certified (NB: DE-CA-18-0005), IEC 62443-4-2 compliant cybersecurity framework
- MOQ Flexibility: 1-unit demo orders for clinics; tiered distributor pricing from 5 units (OEM branding included at no extra cost)
- Logistics Integration: DDP capability to 45+ countries with verified landed cost quotes (includes 2026 EU CBAM carbon fees where applicable)
- Factory Direct Advantage: 19-year manufacturing heritage (est. 2007) with in-house R&D for CBCT/digital sensors – eliminates trading company markups
Why Carejoy passes 2026 due diligence: Their Baoshan District facility (auditable via video) maintains real-time production traceability – each X-ray unit’s component batch numbers are blockchain-verified per MDR Article 27 requirements.
Shanghai Carejoy Medical Co., LTD – Verified 2026 Partner
Core Competency: Factory-direct digital imaging (Panoramic/CBCT), OEM/ODM for global distributors
Verification-First Process: Request pre-shipment regulatory dossier (including 2026 MDR technical documentation) via official channels below:
📧 Email: [email protected] (Reference: “2026 DXR Guide Verification”)
📱 WhatsApp: +86 15951276160 (24/7 technical support)
🏢 Factory Audit: Baoshan District, Shanghai – Virtual/physical audits welcomed with 72h notice
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified case study meeting all outlined criteria; inclusion does not constitute exclusive endorsement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Digital X-ray Units – Key Buying Considerations
Frequently Asked Questions (FAQ) – Digital X-Ray Unit Procurement 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a digital X-ray unit in 2026? | Most modern digital intraoral and panoramic X-ray units operate on standard 100–240V AC, 50/60 Hz, making them compatible with global power systems. However, ensure your clinic’s electrical infrastructure supports stable voltage delivery, especially for cephalometric or CBCT units which may require dedicated circuits. Always confirm voltage compatibility with the manufacturer and consider installing a voltage stabilizer to protect sensitive imaging sensors and processors from power fluctuations. |
| 2. Are spare parts for digital X-ray units readily available, and how does this affect long-term operation? | Availability of spare parts is critical for minimizing downtime. In 2026, prioritize vendors offering guaranteed spare parts support for a minimum of 7–10 years post-discontinuation. Commonly replaced components include X-ray tubes, sensors, position-indicating devices (PIDs), and control panel interfaces. Distributors should maintain regional inventory, and clinics are advised to keep critical spares (e.g., sensor cables, collimators) on hand. Verify if the manufacturer operates certified service centers in your region. |
| 3. What does the installation process for a digital X-ray unit typically involve, and who performs it? | Installation is a certified procedure performed by factory-trained biomedical engineers or authorized technicians. It includes site evaluation (structural support, radiation shielding compliance), electrical setup, unit mounting (wall, floor, or ceiling), software integration with existing practice management systems, and calibration. Regulatory documentation (e.g., radiation safety certificate) must be submitted to local authorities. Turnkey installation packages are standard among premium suppliers and should be included in procurement contracts. |
| 4. What warranty terms should I expect when purchasing a digital X-ray unit in 2026? | Standard warranties cover 2–3 years for the generator, tube head, and control system, with 1–2 years for digital sensors and detectors. Extended warranty options (up to 5 years) are strongly recommended, especially for high-use clinics. Ensure the warranty includes labor, parts, and on-site service response (ideally within 48 hours). Review exclusions carefully—damage from power surges, improper handling, or non-authorized repairs are typically not covered. |
| 5. How do I ensure ongoing service and technical support after the warranty expires? | Post-warranty service plans are essential for lifecycle management. Leading manufacturers offer annual maintenance contracts (AMCs) that include preventive maintenance, priority response, software updates, and discounted spare parts. Confirm service coverage reach, technician qualifications, and SLA (Service Level Agreement) metrics. Distributors should provide multi-lingual technical support and remote diagnostics capabilities, increasingly powered by AI-driven troubleshooting platforms in 2026. |
Need a Quote for Digital Xray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


