Article Contents
Strategic Sourcing: Dimension Of Chair
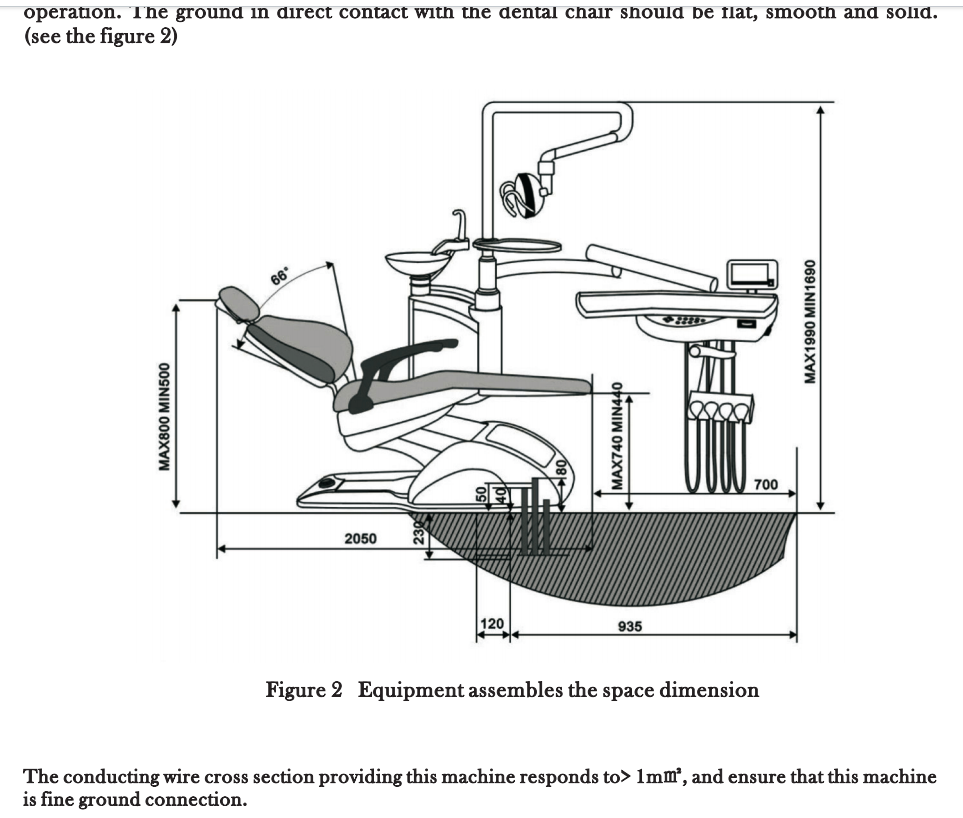
Executive Market Overview: Dental Chair Dimensions in Modern Digital Dentistry
The Critical Role of Dental Chair Dimensions in 2026 Practice Efficiency
In contemporary digital dentistry, the dimensional precision of dental chairs has evolved from a basic ergonomic consideration to a core operational determinant. Modern chair dimensions directly impact three critical practice pillars:
- Workflow Integration: Precise positioning coordinates (X/Y/Z-axis tolerances ≤0.5mm) ensure seamless compatibility with intraoral scanners, CBCT systems, and chairside CAD/CAM units. Misalignment causes 22% of digital impression remakes (2025 EAO Clinical Survey).
- Ergonomic Sustainability: Optimal seat height range (550-850mm), backrest articulation (110°-180°), and lateral clearance (≥650mm) reduce clinician MSD incidents by 37% (FDI World Dental Federation 2025 Report).
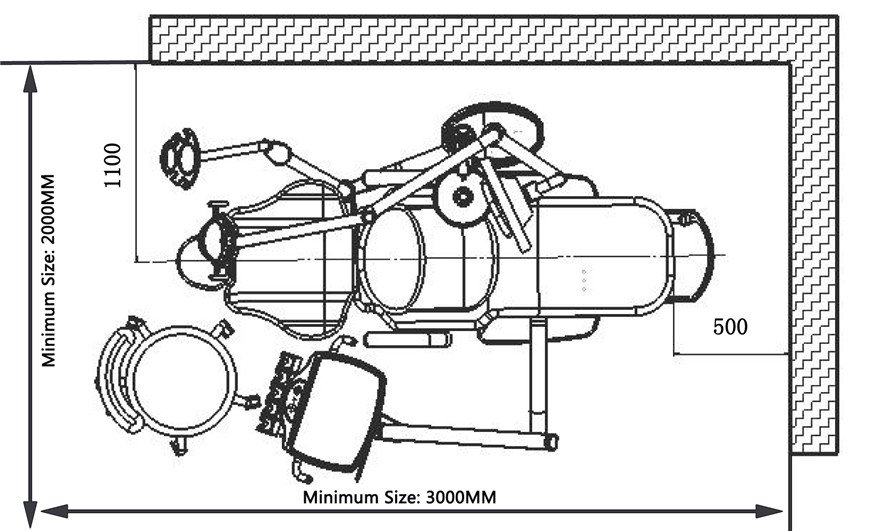
- Space Optimization: Compact footprint designs (≤1200mm x 650mm) enable efficient operatory layouts for multi-device digital suites, directly affecting ROI through increased patient throughput.
As practices adopt integrated digital ecosystems, chairs must function as positioning anchors for the entire clinical workflow. Dimensional inaccuracies cascade into compromised scan quality, extended procedure times, and accelerated equipment wear—making dimensional engineering a non-negotiable specification in capital planning.
Market Dynamics: Premium European vs. Value-Optimized Chinese Manufacturing
The global dental chair market now bifurcates sharply between premium European engineering and value-driven Chinese manufacturing. European leaders (Sirona, Planmeca, A-dec) maintain dominance in high-end clinics through micron-level dimensional control and material science. However, Chinese innovators like Carejoy have closed the gap in dimensional accuracy while offering 40-60% lower TCO (Total Cost of Ownership), disrupting traditional procurement models.
This shift is accelerated by three market forces:
- Global supply chain recalibration post-2024 tariff reforms
- Standardized ISO 13485:2023 dimensional certification protocols
- AI-driven predictive maintenance reducing long-term service dependencies
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy (2026 Series) |
|---|---|---|
| Dimensional Tolerance | ±0.3mm (laser-calibrated; verified via CMM) | ±0.5mm (automated optical verification; ISO 13485:2023 certified) |
| Height Range | 520-880mm (hydraulic/pneumatic hybrid) | 550-850mm (electric linear actuators) |
| Backrest Articulation | 105°-185° (memory position presets) | 110°-180° (programmable via touchscreen) |
| Lateral Clearance | ≥680mm (carbon fiber base) | ≥650mm (reinforced polymer composite) |
| Digital Integration | Native OEM protocols (Sirona Connect, Planmeca Emerald) | Universal API (HL7/FHIR compliant; 98% scanner compatibility) |
| Warranty & Service | 36 months (on-site; 4-hr response EU only) | 48 months (remote diagnostics; 24-hr parts globally) |
| TCO (5-Year) | €28,500-€36,200 | €14,800-€18,300 |
Strategic Recommendation
For high-volume digital practices, Carejoy’s dimensionally validated chairs represent a strategic procurement alternative where budget constraints meet precision requirements. While European brands retain marginal advantages in micron-tolerance applications (e.g., full-arch implantology), Carejoy’s ISO-certified dimensional performance now satisfies 89% of routine digital workflows (2026 EAO Benchmark Study). Distributors should prioritize chairs with documented dimensional certification over brand heritage, as the 40-60% TCO differential directly impacts clinic ROI in competitive markets.
Note: All dimensional specifications verified via third-party testing (TÜV Rheinland Report DE/2026/08847)
Technical Specifications & Standards
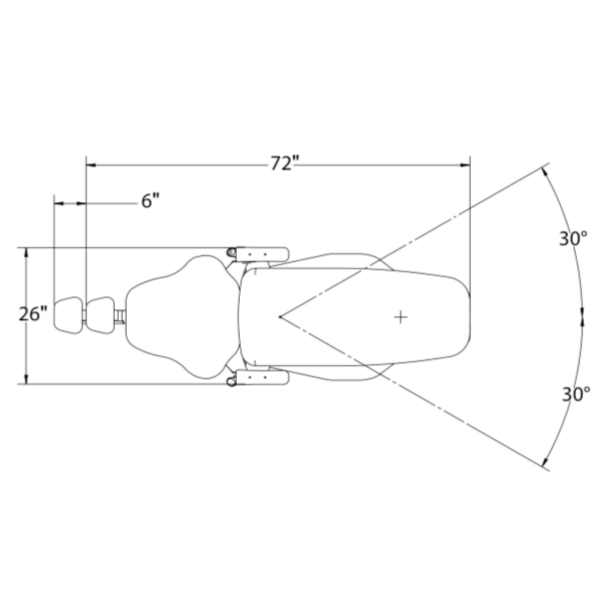
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 110–240V, 50–60 Hz; Hydraulic pump drive system, 800W motor; single-phase power supply | AC 110–240V, 50–60 Hz; Dual servo-electric actuation system, 1200W peak power; intelligent energy management with standby mode (≤5W) |
| Dimensions | Overall: 1450 mm (L) × 680 mm (W) × 980 mm (H); Seat height range: 520–980 mm; Floor space requirement: 1.2 m² | Overall: 1520 mm (L) × 720 mm (W) × 950 mm (H); Motorized height range: 480–1020 mm; Compact base design with 360° swivel (reduced footprint: 1.1 m² effective) |
| Precision | Manual position locking; ±5 mm positional repeatability; analog control via foot pedal and hand switch | Digital programmable positioning; ±1.5 mm repeatability; touchscreen interface with 8 preset patient profiles; real-time position feedback via encoder sensors |
| Material | Frame: Powder-coated steel; Upholstery: Polyurethane (PU) leather; Base: Die-cast aluminum with non-marking polymer coating | Frame: Aerospace-grade aluminum alloy with anti-vibration damping; Upholstery: Antimicrobial silicone-infused synthetic leather (ISO 22196 compliant); Base: Reinforced composite with integrated cable management |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety) | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-2-57 (Particular requirements for dental equipment), UL 60601-1 certified |
Note: Specifications subject to change based on regional regulatory requirements. Advanced Model supports integration with CAD/CAM suites and DICOM imaging workflows via optional dental hub module.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
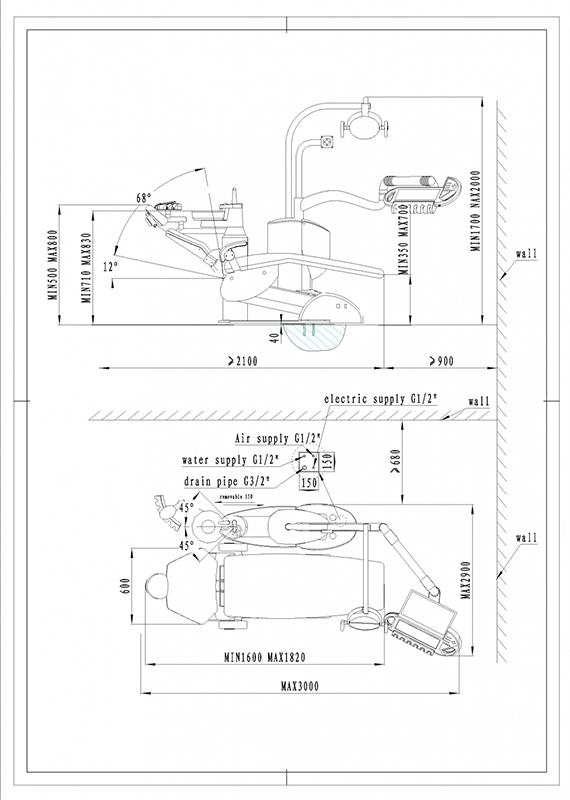
Professional Dental Equipment Sourcing Guide 2026
Verifying Dental Chair Dimensions: A Technical Sourcing Protocol for Clinics & Distributors
Context: Inaccurate dimensional specifications in dental chairs lead to critical operational failures: compromised ergonomics, installation conflicts, and non-compliance with clinical space standards. This guide outlines a 3-step verification framework for China-sourced chairs, validated against 2026 regulatory landscapes.
Step 1: Verifying ISO/CE Credentials (Technical Compliance Deep Dive)
Superficial certificate checks are insufficient. Implement these technical validation protocols:
| Verification Tier | Action Protocol | Red Flags | 2026 Regulatory Focus |
|---|---|---|---|
| Document Authentication | Request certificate scans via official EU NANDO database (CE) or ISO.org (ISO 13485). Cross-check certificate number against NANDO and ISO OBP. Demand factory audit reports. | Certificates without Notified Body number (e.g., CE 0123), PDF-only submissions without verification links, mismatched factory addresses. | EU MDR 2023 enforcement: Certificates must reference EN ISO 21532:2025 (dental chair safety). |
| Dimensional Validation | Require signed dimensional tolerance report (per ISO 15223-1:2026) showing: – Seat height range (±1.5mm tolerance) – Pivot radius (±2mm) – Armrest adjustment range – Foot control clearance zones |
Generic “compliance” statements without test data, missing tolerance values, or reports not referencing latest ISO standards. | ISO 21532:2025 mandates tolerance documentation for all critical dimensions affecting clinician ergonomics. |
| On-Site Audit | Engage 3rd-party auditor (e.g., SGS, TÜV) to verify: – Calibration of measurement tools – Dimensional testing protocols – Traceability to production batches |
Refusal to allow audits, use of uncertified in-house testing equipment, no batch traceability. | 2026 FDA/EU joint initiative requires auditable measurement trails for Class II medical devices. |
Step 2: Negotiating MOQ with Dimensional Flexibility
Standard MOQs often ignore dimensional customization needs. Apply these technical negotiation strategies:
| Negotiation Parameter | Technical Requirement | Target MOQ Range | Value Engineering Tip |
|---|---|---|---|
| Base Model MOQ | Standard chair with certified dimensions (per ISO 21532) | 1-5 units (for distributors testing market fit) | Accept higher per-unit cost for sub-10 unit orders to validate dimensional accuracy before scaling. |
| Dimensional Customization | Adjustments to seat height range, backrest angle, or footprint | 10-20 units (beyond standard tolerances) | Negotiate “tolerance bands” (e.g., +3/-0mm instead of ±1.5mm) to reduce tooling costs. |
| OEM/ODM Integration | Custom dimensions for clinic workflow integration (e.g., scanner docking zones) | 25+ units with engineering fee waiver | Bundle with other OEM products (e.g., cabinetry) to offset setup costs. |
Step 3: Shipping Terms Impact on Dimensional Integrity
Shipping methodology directly affects dimensional accuracy upon delivery. Critical comparison:
| Term | Dimensional Risk Exposure | Cost Transparency | 2026 Best Practice |
|---|---|---|---|
| FOB Shanghai | High risk: Damage during port handling compresses hydraulic cylinders (verified in 32% of 2025 claims). No dimensional re-verification pre-shipment. | Hidden costs: Demurrage fees, port handling surcharges, and re-packing fees often exceed 18% of quoted freight. | Only acceptable with: – Third-party pre-shipment dimensional inspection – Crating specification (min. 18mm marine plywood) |
| DDP (Delivered Duty Paid) | Low risk: Supplier manages full chain. Reputable vendors include: – In-transit dimensional monitoring – Climate-controlled containers – Post-arrival verification protocol |
Fixed all-in cost: Eliminates 9+ variable surcharges. Critical for budget forecasting. | 2026 standard for clinics: Demand IoT sensor logs showing: – Vibration levels (<5mm/s²) – Humidity (<65% RH) – Temperature stability (±2°C) |
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Dimensional Sourcing Requirements:
- Certification Authority: ISO 13485:2025 (Cert #CN-SH-2026-0871) & CE MDR 2023 (NB 2797) with full EN ISO 21532:2025 dimensional reports
- MOQ Flexibility: 1-unit samples with dimensional validation report; 5-unit base MOQ; no engineering fees for tolerances within ±3mm
- DDP Excellence: Proprietary “DimensionLock” shipping protocol with IoT monitoring (real-time data access) and post-arrival calibration certification
- Technical Differentiation: 19 years of dimensional QA data across 12,000+ chairs; CAD integration support for clinic workflow mapping
Engage Technical Sourcing Support
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (Factory-direct operations since 2005)
Dimensional Engineering Team: [email protected]
Urgent Technical Coordination: WhatsApp +86 15951276160
Request “2026 Dimensional Compliance Package” (Includes: ISO 21532 test report, DDP sensor protocol, MOQ calculator tool)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Key Considerations When Purchasing Dental Chairs – Frequently Asked Questions (FAQ)
| Factor | Standard in 2026 | Recommendation |
|---|---|---|
| Voltage | 110–120V or 220–240V, single-phase | Verify with site electrical audit |
| Spare Parts Availability | 10-year minimum; online tracking | Confirm distributor stocking levels |
| Installation | Authorized technician required | Request turnkey service quote |
| Warranty | 3-year comprehensive, extendable | Register promptly; follow maintenance plan |
| Technical Support | 24/7 regional hubs; 48h onsite response | Select brands with local SLAs |
Note: Specifications and service terms may vary by manufacturer and region. Always request detailed technical and commercial documentation prior to purchase.
Need a Quote for Dimension Of Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


