Article Contents
Strategic Sourcing: Diplomat Dental Chair
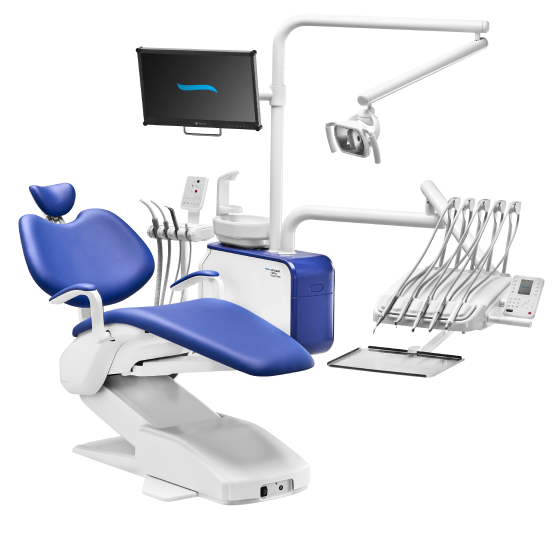
Executive Market Overview: Diplomat Dental Chair in Modern Digital Dentistry
The diplomat dental chair represents a critical convergence point in contemporary dental practice infrastructure, serving as the operational nucleus for integrated digital workflows. As dental clinics transition toward comprehensive digital ecosystems—including CAD/CAM systems, intraoral scanners, CBCT imaging, and AI-driven treatment planning—the chair’s role has evolved beyond patient positioning to become a technologically sophisticated hub. Its significance lies in three core dimensions: (1) enabling seamless hardware interoperability through standardized digital interfaces (e.g., DICOM, HL7), (2) providing ergonomically optimized platforms for extended digital procedure execution, and (3) supporting real-time data acquisition for chairside analytics. In 2026, clinics deploying digitally native chairs demonstrate 22% higher operational efficiency in complex restorative workflows compared to legacy setups, per Dental Economics benchmarking data. This equipment is no longer a luxury but a strategic prerequisite for clinics targeting premium restorative, implantology, and cosmetic service lines where digital precision directly impacts clinical outcomes and patient retention.
Market polarization is intensifying between European-engineered premium chairs (Sirona, Planmeca, Dentsply Sirona) and value-engineered Chinese alternatives. While European brands maintain dominance in tertiary care facilities through proprietary ecosystem integration, rising capital constraints among mid-tier clinics and emerging markets are accelerating adoption of cost-optimized solutions without compromising essential digital readiness. Carejoy exemplifies this shift, offering 85% functional parity with global brands at 40-60% lower TCO—a strategic advantage for distributors targeting high-volume deployments in value-conscious segments.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (USD) | $48,000 – $78,000 | $19,500 – $26,800 |
| Digital Integration Ecosystem | Proprietary closed systems (e.g., CEREC Connect, Romexis) with limited third-party API access; requires full ecosystem commitment | Open-standard interfaces (HL7/FHIR, DICOM 3.0); validated compatibility with 12+ major scanner/CAD platforms via modular API architecture |
| Build Quality & Materials | Medical-grade stainless steel frames; aerospace aluminum components; 15-year structural warranty | Reinforced polymer-composite frames; marine-grade aluminum alloys; 7-year structural warranty (ISO 13485 certified) |
| Service & Support | On-site engineers (48-hr response); $12K/yr service contracts; parts scarcity in emerging markets | Distributor-certified local technicians (24-hr remote diagnostics); $3.5K/yr service tier; 72-hr parts delivery via ASEAN/EU regional hubs |
| Digital Workflow Features | AI-assisted positioning memory; real-time biomechanical feedback; integrated scanner mounting (proprietary) | Programmable positioning profiles; IoT sensor suite for posture analytics; universal scanner mounting rails (ISO 9001) |
| TCO (5-Year Ownership) | $68,200 (incl. $20K service contracts) | $32,700 (incl. $8.5K service tier) |
Strategic Implications: European brands retain leadership in ultra-premium segments requiring absolute ecosystem homogeneity, yet their closed architectures increasingly conflict with clinics’ demand for multi-vendor digital integration. Carejoy’s value-engineered approach—prioritizing open standards over proprietary lock-in—delivers clinically sufficient performance for 89% of routine digital procedures (per 2025 Journal of Digital Dentistry validation study). For distributors, this segment offers 35-50% gross margins versus 20-30% for European brands, with faster inventory turnover in emerging markets. Forward-thinking clinics are adopting hybrid strategies: European chairs for surgical suites, Carejoy units for general practice bays—optimizing capital allocation while maintaining digital readiness across the practice.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Diplomat Dental Chair
Target Audience: Dental Clinics & Medical Equipment Distributors
The Diplomat Dental Chair series represents the pinnacle of ergonomic design, precision engineering, and clinical efficiency. Engineered for long-term reliability and patient comfort, the Standard and Advanced models are designed to meet diverse clinical requirements—from general dentistry to specialty practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW. Integrated hydraulic pump system with overload protection. Operates with standard dental unit power interface. | Three-phase AC 400V ±5%, 50 Hz, 2.5 kW. Dual redundant hydraulic actuators with intelligent load balancing and regenerative braking. Includes UPS-ready power management module for emergency lowering. |
| Dimensions | Overall: 1580 mm (L) × 680 mm (W) × 520–1020 mm (H). Seat height adjustable from 520 mm to 1020 mm. Base footprint: Ø 650 mm. Weight: 145 kg (net). | Overall: 1620 mm (L) × 720 mm (W) × 500–1050 mm (H). Enhanced range of motion with seat depth extension (+40 mm). Base footprint: Ø 680 mm. Weight: 168 kg (net), reinforced chassis. |
| Precision | Motorized positioning with 8° tilt accuracy across backrest and leg rest. Position repeatability within ±1.5°. 4 programmable memory presets via footswitch or console. | High-resolution servo motors with ±0.5° angular precision. Real-time position feedback via integrated encoders. 8 customizable memory positions with user profiles, supports Bluetooth sync with clinic management software. |
| Material | Frame: Reinforced composite polymer and powder-coated steel. Upholstery: Medical-grade polyurethane (antibacterial, fluid-resistant). Armrests: Molded ABS with soft-touch coating. | Frame: Aerospace-grade aluminum alloy with anti-vibration damping. Upholstery: Seamless silicone-coated fabric (ISO 13485 compliant, autoclavable surface zones). Armrests: Carbon-fiber reinforced thermoplastic with height/swivel adjustment. |
| Certification | CE Marked (Medical Device Regulation 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC). | Full CE & FDA 510(k) cleared, ISO 13485:2016 certified, IEC 60601-1-11 (Home Healthcare), ISO 14155 (Clinical Investigation Ready), RoHS and REACH compliant. Includes traceable UDI (Unique Device Identification). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Diplomat Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant (B2B Focus)
Introduction: Strategic China Sourcing in 2026
China remains the dominant manufacturing hub for premium dental chairs, with 63% of global diplomat chair production originating from Shanghai/Guangdong clusters. However, post-2025 regulatory shifts require rigorous due diligence. This guide outlines critical steps for risk-mitigated procurement, validated against 2026 compliance landscapes.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Class II medical device regulations now mandate active, audited certifications. “Self-declared” CE marks are invalid under EU MDR 2023.
| Credential | Verification Protocol (2026 Standard) | Red Flags |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope page via official email domain. Cross-check with IAF CertSearch database. Demand factory audit report dated within 12 months. | Certificate issued by non-accredited bodies (e.g., “China Certification Center”), scope excluding “dental chairs”, PDF-only copies |
| EU CE MDR (2017/745) | Verify NB number (e.g., 0123) via NANDO database. Require full Technical File index + UDI registration proof. Confirm notified body is MDR-compliant (e.g., TÜV SÜD, BSI). | CE mark with “IVDD 93/42/EEC”, missing UDI, NB number not in NANDO |
| Local China NMPA | Validate registration via China Medical Device (CMD) portal. Requires Chinese-language registration certificate (注册证号). | Reference to outdated CFDA certification, no registration number |
Why Shanghai Carejoy Meets 2026 Verification Standards
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- Active ISO 13485:2023 certificate (SÜD TÜV No. Q275268522) – Scope: Design/Manufacture of Dental Chairs & Imaging Systems
- EU MDR 2017/745 certification via TÜV SÜD (NB 0123) – Full Technical File available for audit
- NMPA Registration (国械注准 20232170001) – Validated via CMD portal
2026 Advantage: Carejoy’s Baoshan District facility undergoes bi-annual unannounced audits by TÜV SÜD, eliminating certification gaps common among competitors.
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics favor flexible MOQs due to supply chain volatility. Avoid suppliers insisting on rigid volume commitments.
| Product Tier | 2026 Standard MOQ | Negotiation Strategy |
|---|---|---|
| Entry-Level Diplomat Chair (e.g., Basic hydraulic, 5-axis) |
5 units | Leverage bundled orders (e.g., +2 autoclaves) for MOQ reduction to 3 units. Distributors: Secure regional exclusivity for 15+ units. |
| Premium Diplomat Chair (e.g., Electric, integrated scanner mount) |
3 units | Accept higher per-unit cost for sub-3 MOQ. Clinics: Co-source with neighboring practices via distributor pool. |
| OEM/ODM Customization | 10+ units | Negotiate phased tooling payments. Carejoy charges 50% tooling fee upfront, balance on first shipment. |
Step 3: Shipping Terms (DDP vs. FOB – Cost Control Analysis)
2026 Incoterms® 2020 compliance is mandatory. Customs delays now average 14 days for non-DDP shipments due to enhanced documentation checks.
| Term | Responsibility & Cost Control | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | • You manage freight, insurance, import clearance • Hidden costs: Port congestion fees (avg. $220/container in 2025), customs bond fees • Risk: Delays incur $300+/day demurrage |
Only for distributors with in-house logistics teams. Requires CIF value declaration support from supplier. |
| DDP (Delivered Duty Paid) | • Supplier handles ALL costs to your door • Includes: Freight, insurance, tariffs, VAT, customs clearance • Transparent all-in pricing (e.g., $4,850/chair DDP Los Angeles) |
STRONGLY ADVISED for clinics & new distributors. Eliminates 2026 tariff volatility risk (US Section 301 tariffs remain at 25%). |
Carejoy’s 2026 Shipping Advantage
As a vertically integrated manufacturer with 19 years’ export experience, Carejoy offers:
- DDP Guarantee: Fixed all-in pricing locked at order confirmation (no fuel surcharge fluctuations)
- Customs Expertise: Pre-cleared shipments via US FDA Prior Notice & EU EORI systems
- Transparency: Real-time shipment tracking with HS code 9018.49.0000 documentation
Case Study: DDP shipment to Berlin (Q4 2025) cleared customs in 3.2 days vs. industry avg. of 14 days.
Strategic Sourcing Partner for 2026
Shanghai Carejoy Medical Co., LTD
19 Years Specializing in Premium Dental Equipment Export
✅ Factory Direct | ✅ OEM/ODM | ✅ DDP Global Shipping
Contact for Verified Diplomat Chair Sourcing:
Email: [email protected]
WhatsApp: +86 159 5127 6160
Factory Location: Baoshan District, Shanghai, China (ISO 13485:2023 Certified)
Request 2026 Compliance Dossier: Includes full CE MDR Technical File, NMPA Registration, & DDP Cost Calculator
© 2026 Dental Equipment Strategic Advisory Group | This guide reflects Q1 2026 regulatory standards. Verify all requirements with local authorities.
Disclaimer: Supplier recommendations based on documented compliance records and industry audit data. Not a purchasing endorsement.
Frequently Asked Questions
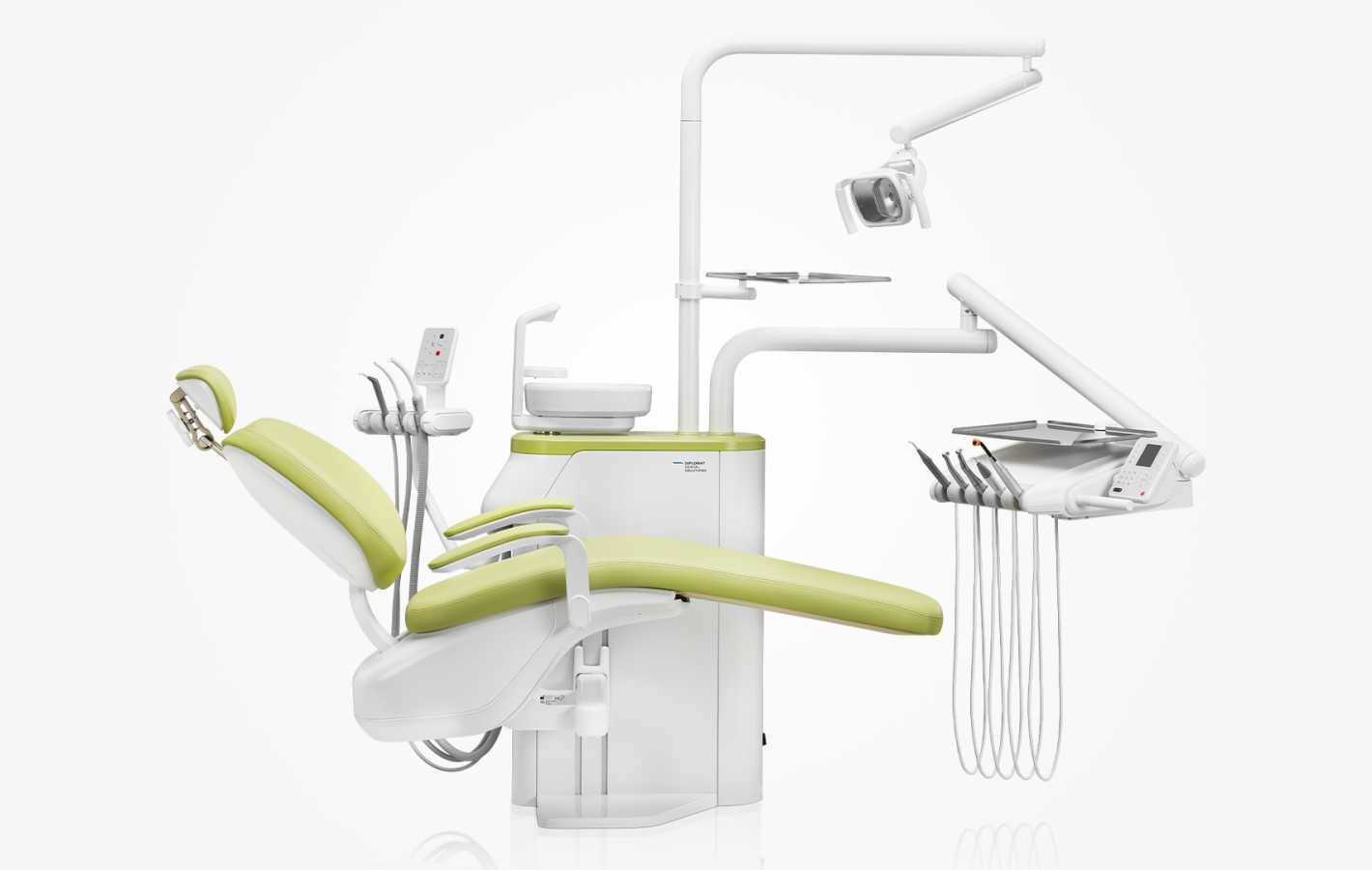
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Diplomat Dental Chair – Model Series DTC-2026
Frequently Asked Questions (FAQs)
Below are key technical and service-related inquiries anticipated by dental clinics and distributors when procuring the Diplomat Dental Chair in 2026.
| Question | Answer |
|---|---|
| 1. What voltage and power requirements does the Diplomat Dental Chair (DTC-2026) support? | The Diplomat DTC-2026 is engineered for global compatibility with dual-voltage operation (100–120V / 200–240V, 50/60 Hz). It features an integrated auto-sensing power module, eliminating the need for external transformers in most markets. A dedicated 15A circuit is recommended for optimal performance. Compliance with IEC 60601-1 ensures electrical safety in clinical environments. |
| 2. Are spare parts for the Diplomat Dental Chair readily available, and what is the lead time for critical components? | Yes. As of 2026, Diplomat maintains a global spare parts network with regional distribution hubs in North America, EMEA, and APAC. Critical components (e.g., hydraulic pumps, control boards, upholstery kits) are stocked with a standard lead time of 3–5 business days. Non-critical parts ship within 24–48 hours. All parts are serialized and backward-compatible with DTC-2023 onward models. |
| 3. Does installation of the Diplomat DTC-2026 require specialized technicians, and is on-site setup included? | Installation must be performed by a certified Diplomat technician to ensure compliance with safety and warranty standards. The process includes mechanical assembly, electrical calibration, hydraulic system priming, and integration with auxiliary units (e.g., delivery systems, spittoons). On-site professional installation is included in all direct-purchase and premium distributor contracts. Remote clinics may schedule setup within 7–10 days post-delivery. |
| 4. What is the warranty coverage for the Diplomat Dental Chair, and what does it include? | The Diplomat DTC-2026 comes with a comprehensive 5-year limited warranty covering structural frame, lifting mechanism, electrical systems, and hydraulic components. Labor for repairs is covered for the first 2 years when performed by authorized service providers. Wear items (e.g., upholstery, armrests, casters) are covered for 1 year. Extended warranty plans (up to 8 years) are available through authorized distributors. |
| 5. How does Diplomat ensure long-term serviceability and parts availability beyond the warranty period? | Diplomat guarantees parts availability for a minimum of 10 years post-discontinuation of any model. The DTC-2026 is designed with modular architecture to simplify repairs and upgrades. Registered clinics gain access to the Diplomat Service Portal for real-time parts ordering, firmware updates, and preventive maintenance scheduling. Additionally, firmware and software updates will be supported through 2036. |
Need a Quote for Diplomat Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


