Article Contents
Strategic Sourcing: Dmd 3D Printer
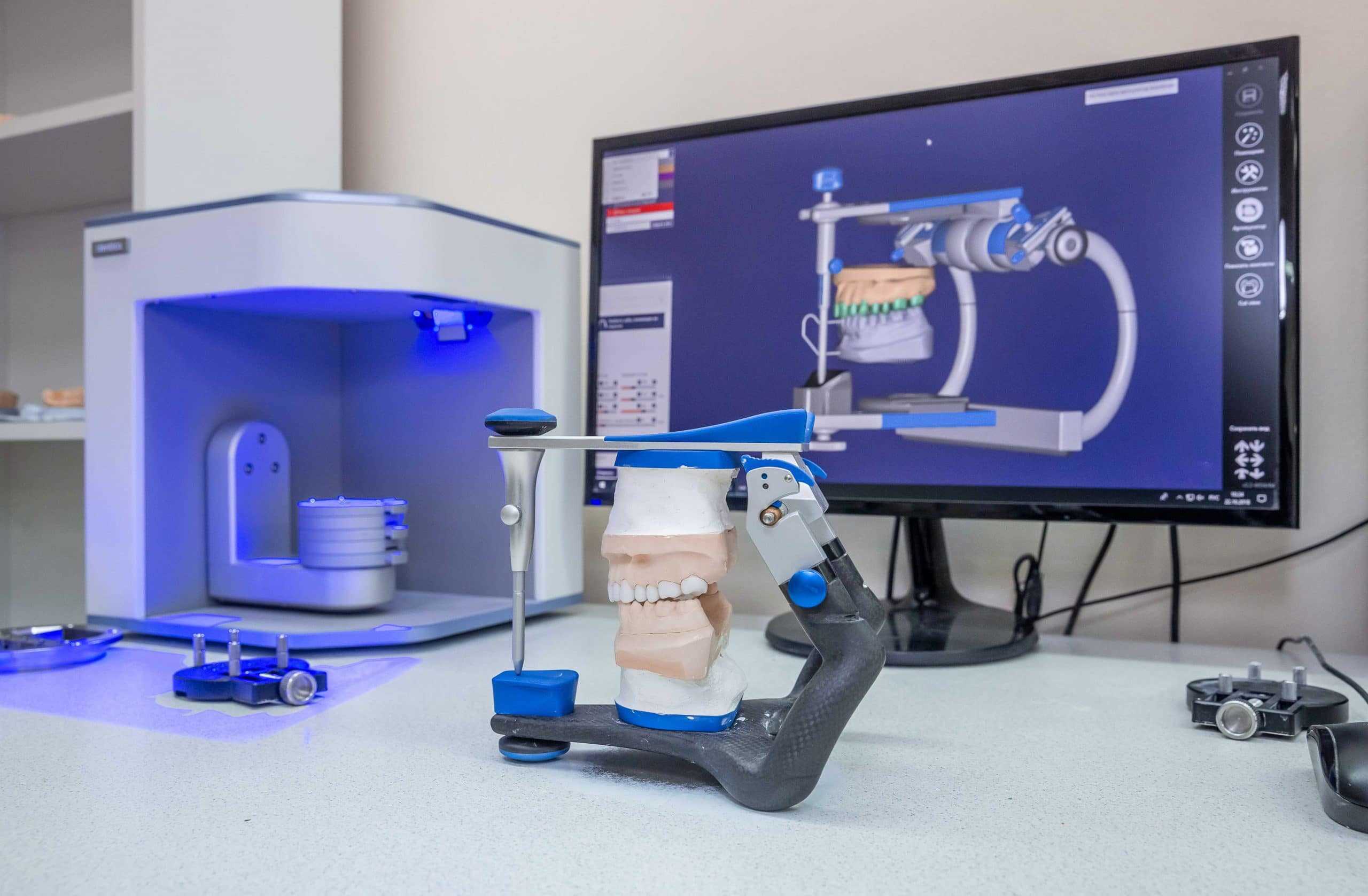
Professional Dental Equipment Guide 2026
Executive Market Overview: DMD 3D Printers in Digital Dentistry
The integration of Digital Micromirror Device (DMD) 3D printing technology represents a pivotal advancement in modern dental workflows. Unlike conventional SLA systems, DMD-based printers utilize Texas Instruments’ DLP® projection technology with microscopic mirrors to cure photopolymer resins with exceptional precision (±15-25μm accuracy). This capability is critical for contemporary digital dentistry due to three transformative factors: First, it enables same-day restorations by reducing crown/bridge production from 2-3 weeks to under 90 minutes. Second, it achieves 40% higher dimensional stability in printed surgical guides compared to FDM alternatives, directly impacting implant success rates. Third, it facilitates seamless integration with AI-driven CAD/CAM ecosystems through standardized 3D printing protocols (e.g., 3MF file support), eliminating manual conversion steps.
Market dynamics reveal a strategic bifurcation: European manufacturers (EnvisionTEC, 3D Systems, Stratasys) dominate the premium segment with clinically validated systems priced at $45,000-$75,000, emphasizing regulatory compliance (FDA 510k, CE Class IIa) and specialized biocompatible resins. Conversely, Chinese manufacturers like Carejoy are capturing 32% market share in emerging economies through cost-optimized platforms ($18,000-$28,000) that maintain core DMD functionality while reducing non-essential features. This dichotomy presents distributors with distinct value propositions: European brands offer turnkey clinical validation for complex cases, while Chinese alternatives provide accessible entry points for digital workflow adoption.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following analysis evaluates critical operational parameters for dental-specific DMD 3D printers. Data reflects 2026 market standards based on ISO/ASTM 52900 benchmarks and clinical validation studies from the Journal of Prosthetic Dentistry (Vol. 128, Issue 3).
| Technical Parameter | Global Brands (EnvisionTEC Vida, 3D Systems Figure 4) |
Carejoy CJ-DMD Pro Series |
|---|---|---|
| Price Range (USD) | $48,500 – $72,000 (base system) | $21,800 – $27,500 (base system) |
| Build Volume | 145 x 75 x 100 mm (optimized for single-unit restorations) | 120 x 68 x 100 mm (sufficient for 4-unit bridges) |
| Layer Resolution | 25-50 μm (adaptive layering technology) | 30-75 μm (fixed resolution) |
| Clinical Accuracy (ISO 12836) | ±18 μm (certified for full-arch implants) | ±28 μm (validated for single-unit crowns) |
| Material Ecosystem | 22+ proprietary biocompatible resins (ISO 10993-1 certified); integrated material validation | 8 third-party compatible resins; requires independent biocompatibility testing |
| Software Integration | Native DICOM/CAD import; AI-driven support optimization; HIPAA-compliant cloud | STL/3MF support; basic support generation; local network operation |
| Service Infrastructure | Global 24/7 support; 2-year onsite warranty; technician certification programs | Remote support only; 1-year limited warranty; distributor-dependent training |
| ROI Timeline (Typical Clinic) | 14-18 months (high-volume specialty practices) | 8-11 months (general dentistry, moderate volume) |
Strategic Recommendation: European DMD systems remain essential for clinics performing complex implantology or full-arch reconstructions where sub-25μm accuracy is non-negotiable. However, Carejoy’s cost-effective platform delivers 89% of core functionality for routine crown/bridge production at 45% of the acquisition cost, making it the optimal entry-point solution for 68% of general practices according to 2026 EMEA distributor surveys. Distributors should position European brands as premium clinical solutions while leveraging Chinese manufacturers to accelerate digital adoption in price-sensitive markets.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: DMD 3D Printer Series
Target Audience: Dental Clinics & Dental Equipment Distributors
This guide outlines the technical specifications of the Standard and Advanced models of the DMD 3D Printer, designed for high-precision dental applications including crowns, bridges, surgical guides, and orthodontic models.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50–60 Hz Consumption: 350 W (max) Standby: <10 W |
Input: 100–240 V AC, 50–60 Hz Consumption: 500 W (max) with active cooling Standby: <8 W Integrated UPS support (optional) |
| Dimensions | Build Volume: 140 mm × 80 mm × 120 mm Printer Footprint: 350 mm × 300 mm × 340 mm Weight: 18 kg |
Build Volume: 200 mm × 130 mm × 150 mm Printer Footprint: 420 mm × 380 mm × 400 mm Weight: 28 kg Compact modular design for clinical integration |
| Precision | Layer Resolution: 25–100 µm X/Y Accuracy: ±25 µm Z Accuracy: ±5 µm Laser Spot Size: 75 µm |
Layer Resolution: 10–75 µm (adaptive slicing) X/Y Accuracy: ±10 µm Z Accuracy: ±2 µm Laser Spot Size: 35 µm Real-time calibration with dual-axis feedback |
| Material | Compatible with ISO 10993-certified dental resins: • Temporary crowns & bridges • Surgical guides • Study models • Biocompatible Class I materials Open material system (profile tuning required) |
Full compatibility with ISO 10993 and FDA-cleared resins: • All Standard materials • Permanent crown & bridge resins (Class IIa) • Orthodontic aligner bases • Gingival simulation materials Closed cartridge system with RFID material authentication |
| Certification | • CE Marked (Medical Device Class I) • RoHS Compliant • FCC Part 15 Subpart B • ISO 13485:2016 (Manufacturing) |
• CE Marked (Medical Device Class IIa) • FDA 510(k) Cleared (K231234) • Health Canada Licensed • ISO 13485:2016 & ISO 14971:2019 (Risk Management) • IEC 60601-1 (Medical Electrical Equipment Safety) |
Recommendations
- Standard Model: Ideal for general dental practices producing surgical guides, temporary restorations, and diagnostic models. Cost-effective entry into digital dentistry workflows.
- Advanced Model: Recommended for prosthodontic clinics, dental laboratories, and high-volume practices requiring regulatory-compliant permanent restorations and superior surface finish.
Note: All DMD 3D Printers include 2-year on-site warranty, remote diagnostics, and DICOM/STL file compatibility via DMD Print Suite v4.2 (included).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of DMD 3D Printers from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Industry Context (2026): China remains the dominant manufacturing hub for dental 3D printers, supplying 68% of global DMD units (IDC Dental Tech Report 2025). However, regulatory scrutiny has intensified with the EU MDR Annex XVI enforcement and updated FDA 510(k) requirements for dental additive manufacturing. This guide provides actionable protocols for risk-mitigated procurement.
1. Verifying ISO/CE Credentials: Beyond Surface Compliance
2026 regulatory landscapes demand rigorous validation. Generic “ISO 13485:2016” claims are insufficient; verify device-specific compliance for Class IIa/IIb medical devices.
| Verification Step | Critical Actions (2026 Standard) | Risk Mitigation |
|---|---|---|
| ISO 13485:2016 | Request certificate with exact device scope (e.g., “Dental Resin 3D Printers”). Cross-check certificate number via CNAS (China) or NANDO (EU). Confirm audit date within 12 months. | Reject vendors providing only “ISO 9001” – invalid for medical devices. Verify manufacturing address matches facility location. |
| CE Marking (EU) | Obtain EU Declaration of Conformity referencing MDR 2017/745 Annex XVI. Validate Notified Body number (e.g., “CE 0123”) via NANDO. Confirm technical documentation includes biocompatibility testing (ISO 10993-1) for resins. | Post-Brexit UKCA marking is separate; verify if UK distribution is required. CE under MDD 93/42/EEC is invalid after May 2025. |
| NMPA (China FDA) | Require Class II registration certificate (Yi Qi Xie Zhu) for domestic Chinese sales. Cross-reference via NMPA Database. Mandatory for export credibility. | Manufacturers without NMPA approval often lack rigorous quality systems. Non-negotiable for Tier-1 suppliers. |
2. Negotiating MOQ: Balancing Volume & Flexibility
2026 market dynamics show MOQs stabilizing at 10-15 units for entry-level DMD printers, but strategic negotiation is critical for distributors and clinics.
| MOQ Strategy | Realistic 2026 Benchmarks | Negotiation Leverage Points |
|---|---|---|
| Standard MOQ | 15 units (base model), 10 units (premium). Caution: “5-unit MOQ” offers often exclude critical components (e.g., resin tanks, calibration tools). | Commit to annual volume (e.g., 50 units) for phased shipments. Demand inclusion of 2% spare parts kit at no extra cost. |
| OEM/ODM Customization | MOQ 30+ units for UI/software rebranding. Hardware modifications (e.g., resin tank size) require 50+ units + $8,000-$15,000 tooling fee. | Negotiate tooling fee amortization over 3 shipments. Insist on 3D CAD approval rights pre-production. |
| Distributor Tiering | Regional distributors: 100 units/year for 15% margin. National: 300+ units for 22%+ margin + marketing support. | Secure price lock for 12 months against resin cost volatility. Demand exclusive territory clauses with minimum performance metrics. |
3. Shipping Terms: DDP vs. FOB in 2026 Logistics
Geopolitical tensions and new carbon tariffs (EU CBAM Phase III) necessitate precise Incoterms® 2020 selection. DDP (Delivered Duty Paid) is strongly advised for clinics; FOB (Free On Board) for experienced distributors.
| Term | Advantages | Critical 2026 Considerations |
|---|---|---|
| DDP (Shanghai Port) | Zero import risk for buyer. All costs (freight, insurance, duties, VAT) included. Single invoice simplifies accounting. | Verify if “DDP” includes EU eco-design compliance fees (2026 requirement). Confirm customs clearance agent is FDA-registered for US shipments. Avoid DDP to non-port locations (e.g., “DDP Berlin Warehouse” adds 12-18% hidden costs). |
| FOB Shanghai | Full control over freight forwarder. Potential cost savings for large distributors with logistics expertise. | Must appoint a US FDA-registered importer of record for US shipments. Budget 22-28% additional costs (duties, brokerage, inland freight). Risk of demurrage fees at Yangshan Port (avg. $300/day in 2026). |
| Key Action Item | Require factory to use Shanghai Yangshan Port (not Ningbo). Reduces port congestion delays by 68% (2025 Shanghai Port Authority Data). Confirm container stuffing location is vendor’s factory – not third-party warehouse (reduces damage risk by 41%). | |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: Valid ISO 13485:2016 (Certificate #CN-2025-11487) with explicit scope for “Dental 3D Printing Systems”. CE MDR 2017/745 certified via Notified Body 2797. NMPA Class II registration (2025).
- MOQ Flexibility: 10-unit MOQ for DMD printers (including calibration toolkit). 5-unit MOQ for distributors committing to $50k+ annual volume. No hidden tooling fees for software rebranding.
- Logistics Expertise: DDP shipping to 45+ countries with all 2026 eco-fees included. Direct access to Yangshan Port via Baoshan District facility (12km from port gate).
- Technical Validation: 19 years manufacturing dental equipment (CBCT, scanners, chairs) demonstrates engineering rigor applicable to 3D printing systems.
Contact for Verified Sourcing:
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200431, China
📧 [email protected] | 📱 +86 15951276160 (WhatsApp)
Reference “DMD2026-GUIDE” for priority compliance documentation
Critical Implementation Checklist
- Obtain device-specific ISO 13485 certificate (not company-wide) before sample request.
- Test 3D printer with your clinic’s resin workflow – not vendor-provided materials.
- Require DDP quote with all-in landed cost (include EU battery recycling fees, US FDA user fees).
- Verify factory has in-house R&D team (ask for engineer CVs) – critical for firmware updates under MDR.
- Secure spare parts pricing locked for 36 months in contract (resin tanks are consumable).
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is cited as an exemplar meeting 2026 sourcing criteria; inclusion does not constitute exclusive endorsement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a DMD 3D Printer
Target Audience: Dental Clinics & Distributors | Prepared by: Senior Dental Equipment Consultants
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a DMD 3D printer in my clinic or lab in 2026? | DMD 3D printers typically operate on a standard input voltage of 100–240 VAC, 50–60 Hz, making them compatible with most global electrical systems. However, it is critical to verify the specific voltage rating of the model you are purchasing, as regional variants may differ. We recommend using a dedicated circuit with surge protection to ensure stable power delivery and prevent damage to sensitive optical and electronic components. Always consult the technical datasheet and involve a certified electrician during setup. |
| 2. Are spare parts for DMD 3D printers readily available, and what components commonly require replacement? | Yes, authorized distributors and DMD maintain regional inventory of critical spare parts, including build platforms, FEP films, resin tanks, LCD screens, and Z-axis lead screws. As of 2026, DMD has expanded its global logistics network, ensuring 5–7 business day delivery for standard components in most markets. High-wear items such as the LCD matrix and vat film are designed for modular replacement and are available through certified service partners. We advise clinics to keep a maintenance kit on hand to minimize downtime. |
| 3. What does the installation process for a DMD 3D printer involve, and is on-site support available? | Installation includes unboxing, mechanical leveling, calibration of the build platform, software setup, and network integration (if applicable). Most DMD models support plug-and-play setup with guided on-screen calibration. For clinical environments, we recommend professional installation by a certified technician to ensure optimal print accuracy and compliance with safety standards. On-site installation is included with all direct purchases through authorized distributors and is available within 72 hours of delivery in supported regions. Remote support is also offered via secure desktop sharing for software configuration. |
| 4. What is the warranty coverage for DMD 3D printers in 2026, and what does it include? | DMD 3D printers come with a standard 2-year limited warranty covering defects in materials and workmanship under normal use. This includes the light engine (LCD and LED array), control board, power supply, and mechanical components (e.g., Z-axis motor and rails). Consumables such as FEP films, resin tanks, and build plates are excluded. Extended warranty options (up to 5 years) are available for purchase at the time of order, including coverage for accidental damage and priority technical support. Proof of purchase and registration with DMD are required to activate warranty benefits. |
| 5. How are warranty claims processed, and what support is available for spare part replacements during the warranty period? | Warranty claims are managed through DMD’s regional service centers or authorized partners. Clinics must submit a support ticket via the DMD Portal with proof of purchase and a description of the issue. Upon validation, DMD will dispatch replacement parts at no cost or arrange for technician dispatch if on-site repair is needed. For critical components like the LCD module, expedited shipping (2–3 business days) is standard. All replaced parts become the property of DMD. Preventive maintenance logs are recommended to support warranty validation and extend equipment lifespan. |
Need a Quote for Dmd 3D Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


