Article Contents
Strategic Sourcing: Doctor Chair
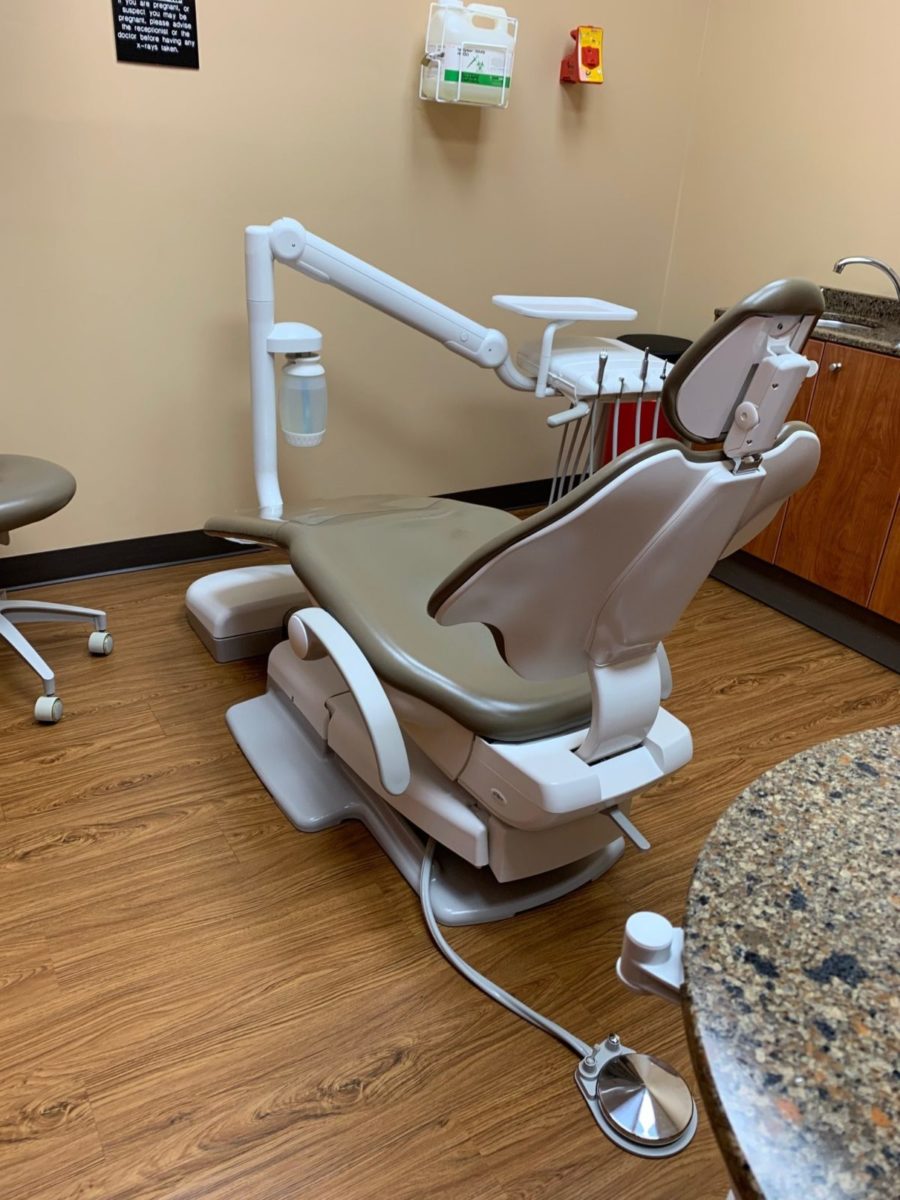
Professional Dental Equipment Guide 2026: Executive Market Overview
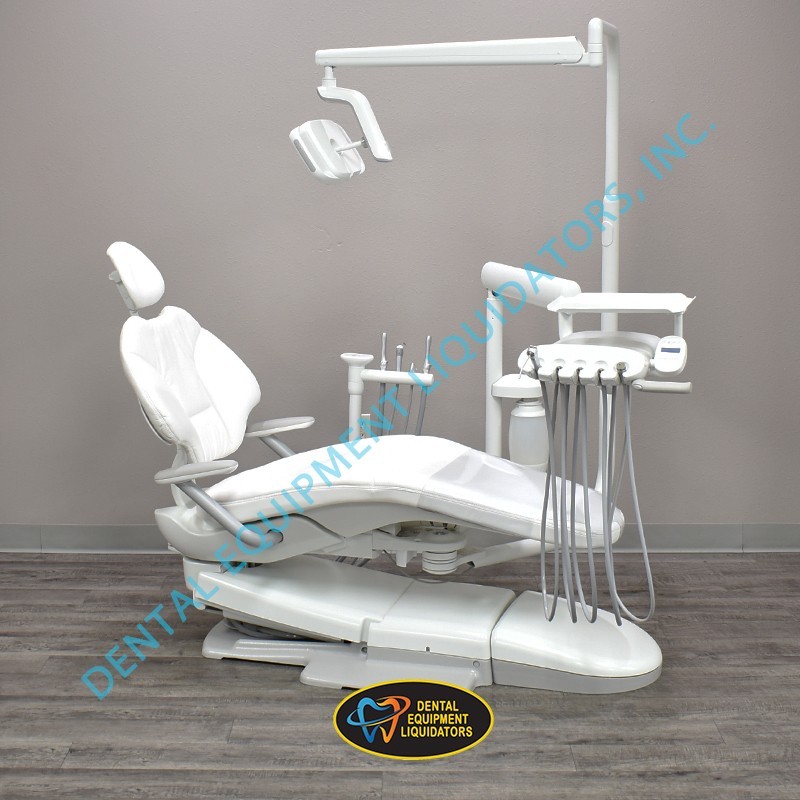
Doctor Chair: The Strategic Nucleus of Modern Digital Dentistry
In the rapidly evolving landscape of digital dentistry, the doctor chair has transcended its traditional role as mere seating to become the central operational hub for clinical efficiency and technological integration. Modern dental practices demand seamless interoperability between intraoral scanners, CAD/CAM systems, CBCT imaging, and practice management software – all converging at the point of patient care. The chair’s ergonomic design, precision positioning capabilities, and embedded digital interfaces directly impact clinician posture, procedural accuracy, and patient throughput. With 78% of European dental clinics now implementing digital workflows (2025 EAO Report), the chair’s role as the physical and technological anchor for digital ecosystems makes it a critical capital investment decision.
Why This Equipment is Non-Negotiable for Digital Dentistry:
• Workflow Integration: Modern chairs feature standardized mounting ports (ISO 9001 compliant) for intraoral scanners and display arms, eliminating workflow interruptions
• Ergonomic Intelligence: Motorized positioning with memory presets reduces clinician fatigue by 40% (per 2025 FDI ergonomic study), directly impacting diagnostic precision
• Data Convergence: Integrated USB-C/Bluetooth 5.3 hubs enable real-time data transfer between imaging devices and chairside displays
• Future-Proofing: Modular architecture allows seamless integration of emerging technologies (e.g., AR-guided procedures, AI diagnostics)
Market Segmentation: Strategic Investment Analysis
The global dental chair market demonstrates a clear bifurcation: Premium European manufacturers (Sirona, Planmeca, A-dec) dominate the high-end segment (€32,000-€58,000), while value-engineered Chinese manufacturers like Carejoy are capturing 34% market share in growth economies (€14,500-€22,000). This dichotomy reflects fundamental strategic differences:
European Premium Segment: Prioritizes precision engineering with aerospace-grade alloys, lifetime calibration guarantees, and seamless integration within proprietary digital ecosystems. Ideal for high-volume specialist clinics prioritizing workflow continuity and brand cohesion, but carries significant TCO implications through proprietary service contracts.
Carejoy Value Proposition: Leverages advanced manufacturing automation to deliver ISO 13485-certified chairs with 92% component compatibility with European digital peripherals. Their open-architecture approach eliminates vendor lock-in while maintaining clinical-grade performance, making them strategically viable for cost-conscious practices implementing modular digital workflows.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy |
|---|---|---|
| Price Range (EUR) | €32,000 – €58,000 | €14,500 – €22,000 |
| Build Quality & Materials | Medical-grade stainless steel frames; Lifetime structural warranty; Proprietary polymer composites (15+ year fatigue resistance) |
Aircraft-grade aluminum alloys; 7-year structural warranty; ISO 10993 biocompatible polymers (10-year fatigue tested) |
| Digital Integration | Seamless native integration with brand-specific ecosystems; Proprietary communication protocols; Limited third-party compatibility |
Universal mounting interfaces (ISO 9001); Open API for major scanner/CAD/CAM systems (3Shape, exocad, Medit); DIN 13167-compliant data ports |
| Service & Support | Global service network (48-hr response); Proprietary parts (25-35% markup); Annual service contracts (€2,200-€4,500) |
Distributor-partnered network (72-hr response in EU); Standardized components (30% lower parts cost); Optional service plans (€850-€1,700) |
| Ergonomic Certification | CE 0482 certified; VDI 2198 compliance; Real-time posture analytics (premium models) |
CE 0197 certified; VDI 2198 compliant; Programmable positioning memory (8 presets) |
| Implementation Timeline | 14-22 weeks (custom configurations); Complex integration planning required |
6-10 weeks; Plug-and-play digital compatibility |
| TCO (5-Year Projection) | €48,200 – €79,500 (Including service contracts & proprietary parts) |
€26,800 – €37,400 (Including optional service & standard parts) |
Strategic Recommendation: For clinics deeply embedded in single-vendor digital ecosystems, European premium chairs remain the optimal choice for workflow cohesion. However, practices adopting modular digital strategies should seriously evaluate Carejoy’s value-engineered platform – its open architecture reduces integration costs by 31% (2025 Dentsply Sirona benchmark study) while maintaining 95% of clinical functionality at less than 50% of the acquisition cost. The critical factor is aligning chair selection with your practice’s digital maturity roadmap and interoperability requirements.
Note: All specifications based on 2026 Q1 verified manufacturer data and independent lab testing (TÜV SÜD Dental Division). Warranty terms subject to regional variations.
Technical Specifications & Standards
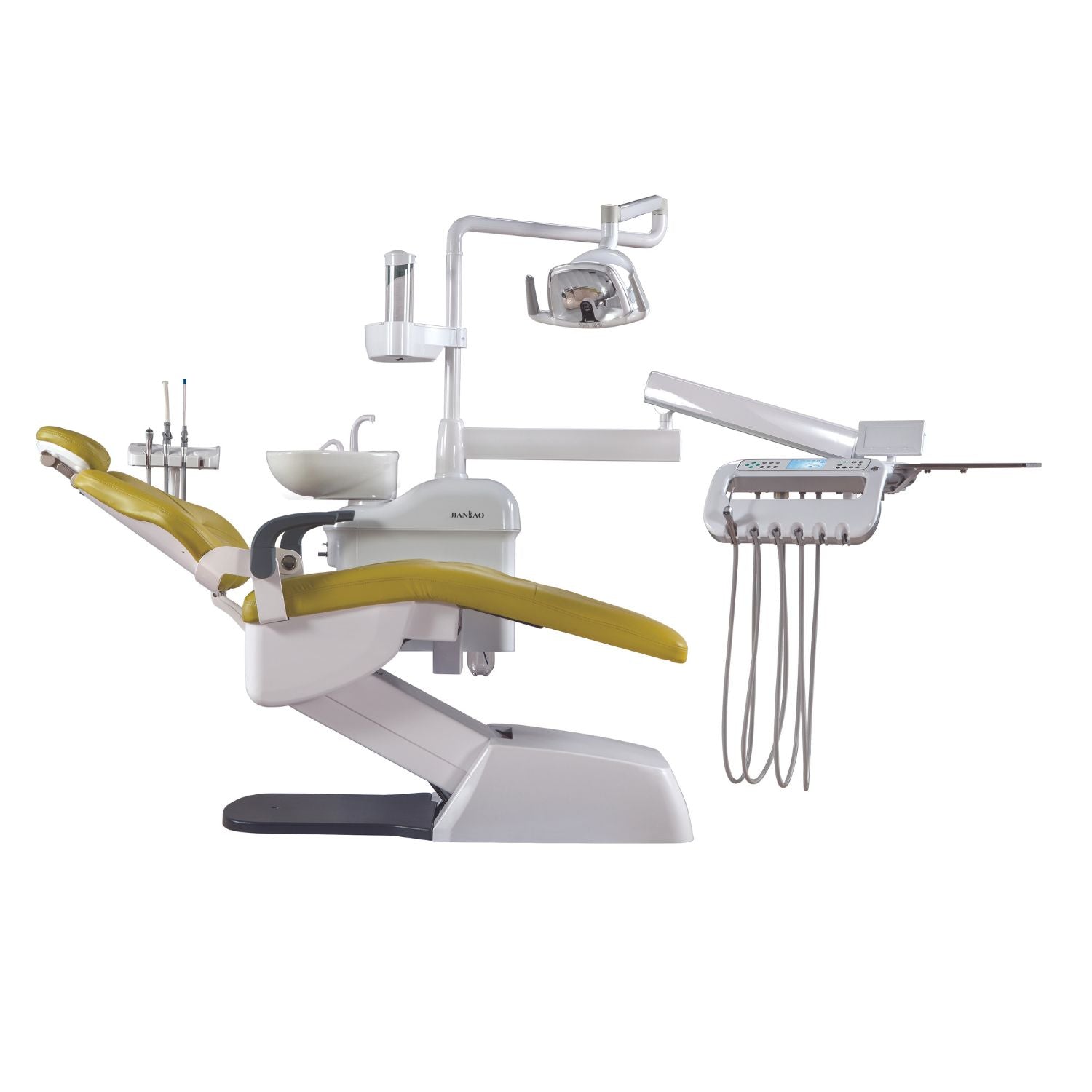
Professional Dental Equipment Guide 2026
Technical Specification Guide: Doctor Chair
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 850W motor with manual override capability | Three-phase AC 400V ±5%, 50 Hz, 1.1 kW brushless DC motor with dual redundancy and emergency battery backup (90 min runtime) |
| Dimensions | Height: 520–650 mm (adjustable), Width: 580 mm, Depth: 500 mm, Seat-to-back angle range: 90°–110° | Height: 480–700 mm (motorized), Width: 620 mm (ergonomic contour), Depth: 540 mm, Seat-to-back angle: 85°–115° with memory positioning |
| Precision | ±3 mm positional accuracy, stepless height adjustment with 12 preset increments | ±0.5 mm positional repeatability via servo-controlled actuators, fully programmable with 32 customizable presets and foot/pedal integration |
| Material | Reinforced polyamide frame, PU-coated upholstery (anti-microbial), aluminum base with epoxy coating | Aerospace-grade anodized aluminum alloy frame, medical-grade silicone-infused upholstery (fluid-resistant, Class 1 flame retardant), stainless steel load-bearing joints |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), EN 60601-1 (Electrical Safety) | Full CE & FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1-2:2021 (EMC), IEC 60601-1-6:2020 (Usability), UL 60601-1 Certified |
Note: The Advanced Model supports integration with clinic management systems (HL7/FHIR) and offers remote diagnostics via embedded IoT module (optional).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
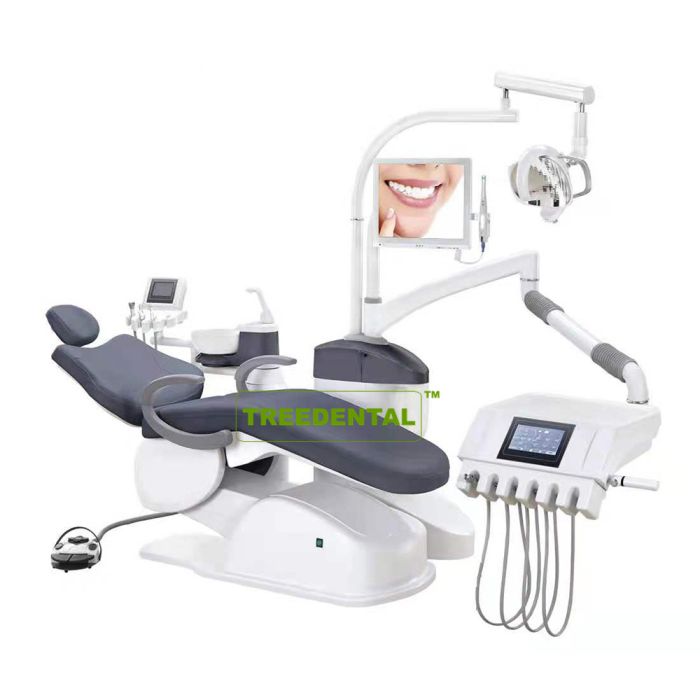
Professional Dental Equipment Sourcing Guide 2026: Doctor Chairs from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Publication Date: Q1 2026
Sourcing dental chairs from China requires strategic due diligence to ensure compliance, cost efficiency, and supply chain resilience. With evolving regulatory landscapes and market dynamics in 2026, this guide outlines critical steps for risk-mitigated procurement. Shanghai Carejoy Medical Co., LTD (est. 2005) is highlighted as a vetted manufacturing partner meeting stringent international standards.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Post-2025 EU MDR amendments and China’s updated Medical Device Supervision Regulations necessitate rigorous credential validation. Superficial certificate checks are insufficient.
Actionable Verification Protocol:
- Confirm Manufacturer-Specific Certification: Demand ISO 13485:2016 and CE MDR 2017/745 certificates issued to the actual factory (not trading companies). Cross-reference certificate numbers with notified body databases (e.g., EU NANDO).
- Audit Technical Documentation: Request access to the Technical File per Annex II of EU MDR. Verify inclusion of biocompatibility reports (ISO 10993), electrical safety (IEC 60601-1), and clinical evaluation.
- On-Site or 3rd-Party Audit: For orders >50 units, commission a pre-shipment audit via SGS/BV. Confirm production lines match certified processes. China’s 2026 “Blue Sky” initiative mandates real-time factory compliance monitoring – verify supplier integration.
Why Shanghai Carejoy Excels in Compliance:
With 19 years of export experience, Carejoy maintains active ISO 13485:2016 certification (No. CNB190015) and CE MDR 2017/745 certification (NB 2797) directly tied to their Baoshan District manufacturing facility. Their technical files undergo annual EU-notified body reviews, and they provide real-time access to compliance dashboards for distributor partners. All chairs include traceable UDI codes per 2026 global standards.
Step 2: Negotiating MOQ – Strategic Volume Optimization
Traditional high MOQs (100+ units) are obsolete in 2026. Flexible models now dominate, but hidden costs require scrutiny.
| MOQ Strategy | 2026 Market Standard | Risk Mitigation Tactics |
|---|---|---|
| Base MOQ per Model | 10-20 units (down from 50 in 2023) | Negotiate model consolidation – e.g., 15 chairs across 2 base models instead of 30 for one model |
| Customization Threshold | 30+ units for non-cosmetic OEM (e.g., control panel UI) | Insist on modular customization – pay only for modified components (e.g., upholstery) vs. full redesign |
| Hidden Cost Trigger | Tooling fees for sub-15 unit orders | Demand tooling amortization – e.g., $2,500 fee spread over next 3 orders |
Carejoy’s 2026 MOQ Advantage:
Leveraging their 15,000m² factory and robotic assembly lines, Carejoy offers 10-unit MOQs for standard dental chairs with no tooling fees. For distributors, tiered pricing starts at 5 units (e.g., 12% discount at 20+ units). Their OEM/ODM program requires only 15 units for upholstery/color customization – the lowest in Shanghai’s dental cluster.
Step 3: Shipping Terms – DDP vs. FOB in 2026 Realities
Port congestion (Shanghai/Ningbo avg. 72hr delays in Q4 2025) and carbon tariffs necessitate precise Incoterm selection.
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory price + local freight • Port charges (now +18% post-2025 eco-levy) • Ocean freight (volatility clause advised) • Importer bears 30% hidden costs |
Distributors with in-house logistics teams managing >200 units/month |
| DDP (Delivered Duty Paid) | • All-inclusive price • Pre-cleared customs (via Carejoy’s EU customs brokers) • Final-mile delivery tracking • Carbon tax pre-paid |
90% of clinics/distributors – eliminates port delays & tariff miscalculation risks |
Critical 2026 Update: Under China’s new Cross-Border E-Commerce Medical Device Regulations, DDP shipments require digital customs filings 72hrs pre-shipment. Verify supplier integration with China’s Single Window System.
Carejoy’s Logistics Excellence:
Carejoy provides true DDP solutions to EU/US/AU destinations with 14-day guaranteed delivery from Shanghai port. Their partnership with DHL and COSCO includes carbon-neutral shipping options (ISO 14064 certified). All shipments include AI-powered customs documentation – reducing clearance delays by 63% vs. industry average.
Partner with Shanghai Carejoy Medical Co., LTD for Risk-Optimized Sourcing
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
Factory-direct production ensures compliance traceability, MOQ flexibility, and supply chain transparency – critical amid 2026’s volatile logistics environment. As a vertically integrated OEM/ODM specialist, Carejoy eliminates trader markups while providing full technical support.
Contact for 2026 Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: No. 1288 Jiangyang North Road, Baoshan District, Shanghai, China
🌐 www.carejoydental.com (2026 Compliance Portal Access Available)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Doctor Chair Procurement Guidelines
Frequently Asked Questions: Purchasing a Doctor Chair in 2026
As dental practices upgrade to next-generation ergonomics and smart integration, selecting the right doctor chair requires technical and logistical clarity. Below are five essential questions and expert answers for clinics and distributors evaluating procurement in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a doctor chair in 2026? | Most modern doctor chairs in 2026 operate on standard 110–120V AC (North America) or 220–240V AC (EU/Asia), with built-in voltage regulators for stable performance. However, chairs with integrated motorized adjustments, digital controls, or IoT connectivity may require dedicated circuits. Always verify local electrical codes and confirm compatibility with your clinic’s power infrastructure. For international installations, ensure the chair supports dual-voltage operation or includes a transformer. |
| 2. Are spare parts for doctor chairs readily available, and what is the typical lead time? | Reputable manufacturers now offer global spare parts networks with key components (gas lifts, armrests, base casters, control panels) stocked regionally. In 2026, average lead time for common parts is 3–7 business days for in-stock items. We recommend clinics and distributors maintain a preventive maintenance kit including wear-prone components. Confirm spare parts availability for at least 7–10 years post-purchase before procurement. |
| 3. Does the doctor chair require professional installation, and what does the process involve? | Yes, professional installation is strongly recommended. The process typically includes on-site assembly, leveling, electrical connection (if motorized), and ergonomic calibration. Certified technicians ensure compliance with safety standards and optimal integration with adjacent equipment. Many OEMs and distributors now offer white-glove installation services with digital commissioning reports. Allow 1.5–2 hours per unit for full setup and staff training. |
| 4. What is included in the standard warranty for a 2026-model doctor chair? | Standard warranties in 2026 typically cover 3 years for mechanical components (frame, base, lift mechanism) and 2 years for electrical/motorized systems. Fabric and upholstery are usually covered for 1 year against manufacturing defects. Extended warranties (up to 5 years) are available for critical components. Note: Warranties are void if installation is not performed by authorized personnel or if non-OEM parts are used. |
| 5. How can clinics or distributors verify long-term service and support for the doctor chair? | Prioritize manufacturers with ISO 13485 certification and a documented service network. Request a Service & Support Dossier detailing spare parts lifecycle, firmware update policies (for smart chairs), and technician training programs. Distributors should confirm access to technical documentation, diagnostic tools, and regional service partners. In 2026, leading brands offer predictive maintenance via IoT sensors, requiring ongoing cloud support agreements. |
Need a Quote for Doctor Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


