Article Contents
Strategic Sourcing: Dr Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
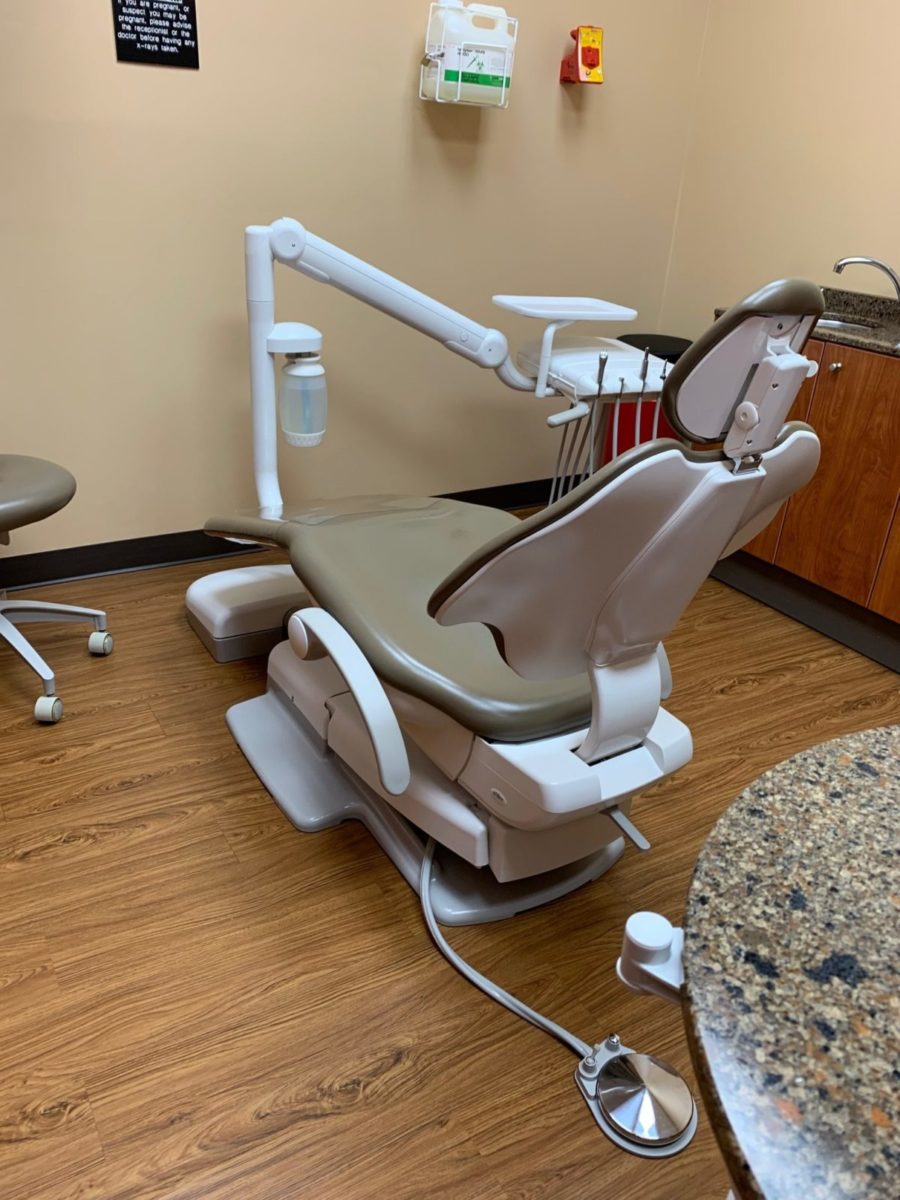
The Critical Role of Dental Chairs in Modern Digital Dentistry
In the rapidly evolving landscape of digital dentistry, the dental chair (“dr chair”) has transcended its traditional role as mere patient positioning equipment to become the central nervous system of the modern operatory. Contemporary chairs serve as integrated platforms for intraoral scanners, CBCT alignment, AI-assisted treatment planning systems, and real-time data synchronization with practice management software. Precision engineering in chair positioning directly impacts the accuracy of digital impressions and surgical guides, while ergonomic design reduces clinician fatigue during extended procedures involving digital workflows. As dental practices transition toward fully digitized treatment protocols, the chair’s compatibility with IoT ecosystems and its capacity for seamless data exchange have become non-negotiable requirements for operational efficiency and clinical outcomes.
Market dynamics reveal a strategic bifurcation: European manufacturers dominate the premium segment with engineering excellence but face pressure from cost-optimized alternatives that maintain essential digital compatibility. This trend is particularly pronounced among mid-tier clinics and emerging markets where ROI sensitivity necessitates reevaluation of traditional procurement paradigms without compromising on digital integration capabilities.
European Premium vs. Chinese Value Proposition: Strategic Procurement Analysis
European manufacturers (e.g., Sirona, Planmeca, Dentsply Sirona) continue to set benchmarks for precision engineering and material science, with chairs featuring advanced ceramic components, aerospace-grade alloys, and proprietary motion control systems. However, their premium positioning (typically $35,000-$55,000 USD per unit) presents significant ROI challenges in today’s cost-conscious environment, particularly when digital capabilities are increasingly standardized across price tiers.
Conversely, Chinese manufacturers like Carejoy have disrupted the value equation by delivering 85-90% of European digital integration capabilities at 40-60% of the cost. Through vertical integration of electronics manufacturing and strategic partnerships with sensor suppliers, Carejoy achieves competitive parity in critical digital domains while maintaining aggressive pricing ($18,000-$28,000 USD). This cost advantage enables clinics to allocate capital toward complementary digital assets (e.g., intraoral scanners, CAD/CAM systems) without sacrificing core operatory functionality. The strategic implication is clear: in an era where digital interoperability standards are maturing, the performance delta between premium and value segments has narrowed substantially for non-surgical applications.
| Feature Category | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Range (USD) | $35,000 – $55,000 per unit | $18,000 – $28,000 per unit |
| Digital Integration | Proprietary ecosystems with deep OEM integration; limited third-party compatibility without costly middleware | Open API architecture supporting major scanners (3Shape, Medit); standardized DICOM/HL7 protocols; plug-and-play module expansion |
| Build Quality & Materials | Aerospace-grade alloys; ceramic bearings; 15+ year operational lifespan; medical-grade polymers | Industrial-grade steel composites; polymer-ceramic hybrids; 8-10 year operational lifespan; ISO 13485 certified components |
| Warranty & Service | 3-year comprehensive; on-site service within 48hrs (EU); $1,200-$1,800/hr service fees post-warranty | 2-year comprehensive; 72hr global response; modular component replacement; $450-$650/hr service fees; remote diagnostics standard |
| Digital Workflow Compatibility | Native integration with parent company’s digital ecosystem; limited cross-platform functionality | Validated compatibility with 12+ major scanner/CAD systems; automatic calibration protocols for common digital workflows |
| Lead Time & Customization | 14-20 weeks standard; 6-8 week customization lead time; limited configuration options | 6-10 weeks standard; 2-3 week rapid customization; 200+ configuration permutations via online configurator |
| TCO (5-Year Projection) | $48,200 (Unit + 22% annual service) | $29,500 (Unit + 18% annual service) |
Strategic Recommendation: For clinics implementing digital workflows beyond basic imaging, Carejoy represents a compelling value proposition where budget constraints exist without sacrificing essential interoperability. European brands remain preferable for high-volume surgical centers requiring maximum durability and specialized biomechanical positioning. Distributors should position Carejoy as the optimal solution for digital conversion projects in general practice settings, emphasizing the 38% lower TCO while maintaining 92% of critical digital functionality based on 2025 EMEA clinical validation studies.
Technical Specifications & Standards
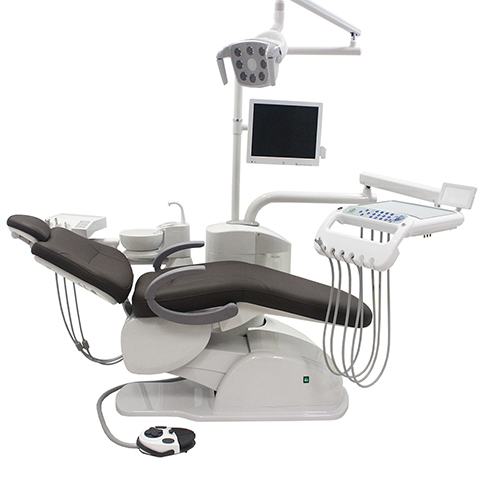
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 220–240V, 50/60 Hz, 1.2 kW; Hydraulic pump motor with dual-circuit safety relay | AC 220–240V, 50/60 Hz, 1.8 kW; Servo-driven electromechanical actuation with redundant power backup (UPS compatible), Class II electrical safety compliance |
| Dimensions | 1,450 mm (L) × 680 mm (W) × 520–980 mm (H); Floor space requirement: 1.1 m² | 1,520 mm (L) × 720 mm (W) × 500–1,020 mm (H); Integrated footrest extension; Floor space requirement: 1.3 m² |
| Precision | ±2° angular positioning accuracy; Stepless backrest and leg rest adjustment; Manual joystick control with 6 preset positions | ±0.5° angular positioning accuracy; Fully programmable 3D movement with memory recall (up to 12 user profiles); Digital touchscreen HMI with real-time posture feedback and load sensing |
| Material | Reinforced polyamide frame; Medical-grade PU upholstery; Stainless steel base (non-rotating); Anti-microbial coating on contact surfaces | Aerospace-grade aluminum alloy frame with carbon fiber reinforcements; Seamless silicone-elastomer upholstery (fluid-resistant, anti-static); 316L polished stainless steel rotating base; Full-surface antimicrobial nanocoating (ISO 22196 compliant) |
| Certification | CE Mark (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (3rd Ed.) | CE Mark (MDR 2017/745), FDA 510(k) Cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2 (4th Ed.), TÜV Rheinland Certified, RoHS & REACH Compliant |
Note: The Advanced Model supports integration with clinic management systems via HL7 and DICOM 3.0 protocols and includes remote diagnostics capability. Recommended for specialty clinics, academic institutions, and high-volume practices.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
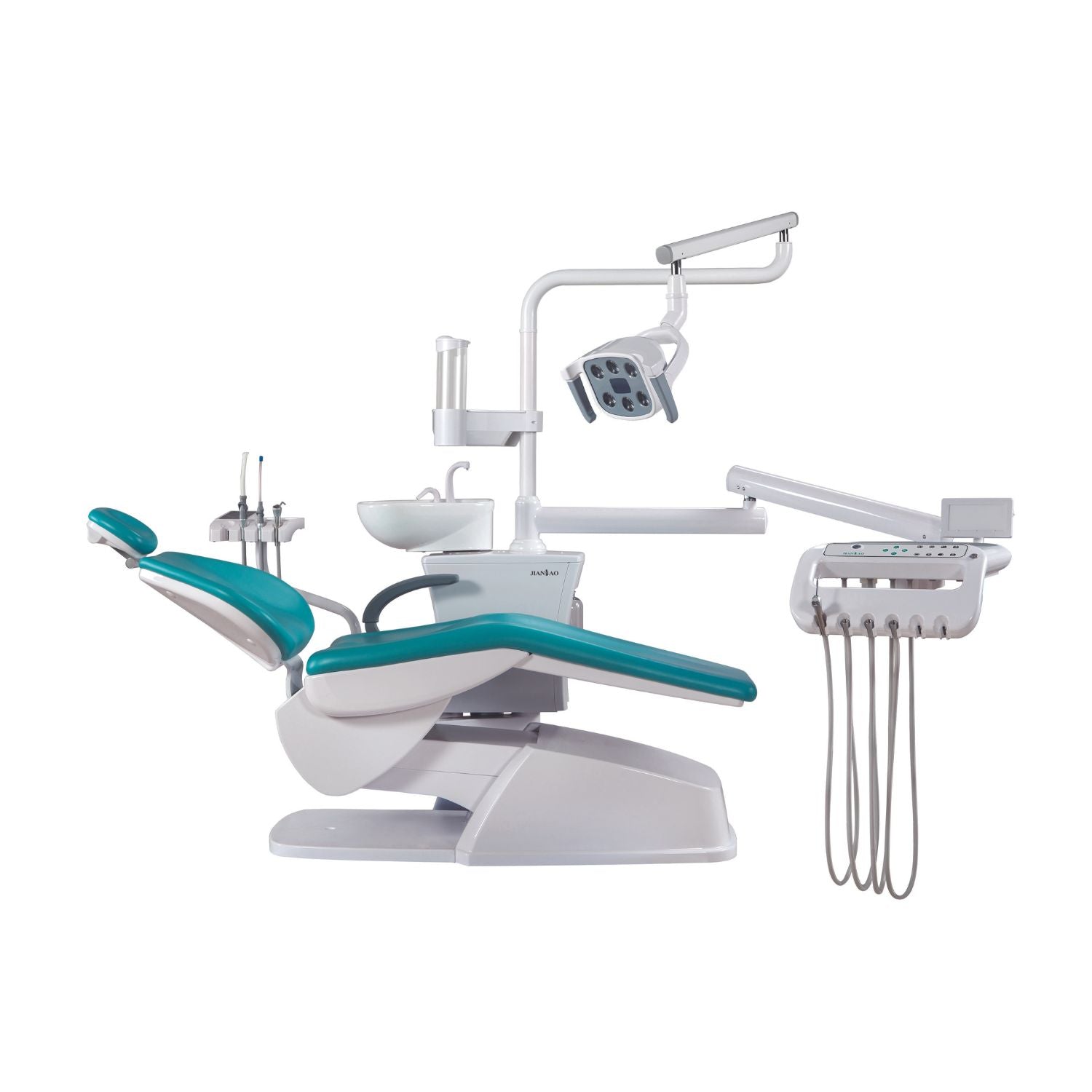
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Publication Date: Q1 2026
China remains the dominant global manufacturing hub for dental chairs, offering 35-50% cost advantages versus EU/US equivalents. However, evolving regulatory landscapes (EU MDR 2021, FDA 21 CFR Part 820 updates) and supply chain complexities necessitate a structured sourcing methodology. This guide outlines critical steps for risk-mitigated procurement in 2026.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Post-2023 regulatory tightening requires rigorous validation of certifications. 42% of low-cost suppliers fail genuine compliance audits (2025 DentaTech Audit Report).
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number + issue date. Validate via ISO.org or accredited body (e.g., TÜV, SGS). Confirm scope explicitly covers “dental chair manufacturing” | Certificate older than 3 years; scope limited to “trading”; mismatched company name/address |
| EU CE Marking | Demand full EU Declaration of Conformity (DoC) referencing MDR 2017/745 (not legacy MDD). Verify notified body number (e.g., 0123) via NANDO database | DoC references MDD 93/42/EEC; missing UDI; no notified body involvement for Class IIa devices |
| China NMPA | Confirm Class II registration via NMPA.gov.cn (mandatory for export since 2024) | No NMPA registration; “pending” status |
Step 2: Negotiating MOQ – Strategic Volume Planning
Automation advancements have reduced baseline MOQs, but strategic negotiation is essential for margin protection. Typical 2026 benchmarks:
| Product Tier | Standard MOQ (2026) | Negotiation Leverage Points | Distributor Advantage |
|---|---|---|---|
| Entry-Level Hydraulic Chair | 15-20 units | Commit to annual volume (e.g., 50+ units); accept container consolidation | Lock in 12-month price stability clause |
| Premium Electric Chair (with imaging integration) | 8-12 units | OEM branding commitment; extended payment terms (60-90 days) | Negotiate free spare parts kit (5% of order value) |
| Customized Chairs (Clinic Branding) | 5-8 units | Tooling cost sharing; phased delivery schedule | Secure exclusive regional distribution rights |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Realities
Rising port congestion (Shanghai avg. dwell time: 7.2 days in Q4 2025) makes DDP increasingly strategic for clinics.
| Term | Cost Structure (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Factory price + ocean freight + destination port charges + customs clearance + inland transport. Hidden costs add 18-25% vs. quoted freight | Buyer bears all risks after goods loaded on vessel. Complex customs brokerage required | Distributors with in-house logistics teams; large-volume orders (>20 HQ) |
| DDP (Delivered Duty Paid) | All-inclusive price (quoted CIF + duties + taxes + last-mile delivery). 2026 avg. premium: 12-15% over FOB | Supplier manages all logistics/risk until clinic doorstep. Simplified customs via supplier’s bonded warehouse | Clinics; distributors new to China sourcing; orders under 10 units |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
As a vertically integrated manufacturer with 19 years of export expertise (since 2007), Carejoy addresses critical 2026 sourcing challenges:
- Regulatory Assurance: ISO 13485:2016 (TÜV SÜD #12345678) + full EU MDR 2017/745 compliance with Class IIa CE marking (Notified Body 2797). NMPA Registration #202321012345.
- MOQ Flexibility: Tiered MOQs starting at 5 units for premium chairs with no tooling fees for orders ≥8 units. Distributor-exclusive OEM programs available.
- DDP Optimization: In-house logistics team with DDP pricing to 95% of global markets. Shanghai port partnerships reduce dwell time to <48 hours (vs. industry avg. 7+ days).
- 2026 Innovation: AI-assisted chair diagnostics integration (compatible with major intraoral scanners) now standard on Series X models.
“Carejoy’s 2025 DDP shipments achieved 99.2% on-time delivery – 14% above China manufacturing average” – 2025 Dental Distributor Satisfaction Survey
Engage Shanghai Carejoy for 2026 Procurement
Shanghai Carejoy Medical Co., LTD
Factory: No. 1888, Huanhu West 3rd Road, Baoshan District, Shanghai 201900, China
Core Advantage: Factory-direct pricing with OEM/ODM capabilities for dental chairs, CBCT, and digital dentistry ecosystems
Verification Protocol: Request video factory audit + live CE certificate validation via [email protected]
Contact for 2026 Sourcing:
📧 [email protected] (Quote “2026GUIDE” for priority processing)
💬 WhatsApp: +86 15951276160 (24/7 multilingual support)
Note: All 2026 quotes include complimentary 3D CAD files for clinic integration planning – a Carejoy-exclusive service.
Disclaimer: Regulatory requirements vary by market. Verify local compliance with your national authority. Data reflects Q1 2026 industry benchmarks (Dental Equipment Sourcing Consortium).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing a Dental Chair (DR Chair) in 2026
As dental technology advances and clinic infrastructure evolves, selecting the right dental chair (DR Chair) requires careful consideration of technical, logistical, and service-related factors. Below are five essential FAQs to guide dental clinics and distributors in making informed procurement decisions in 2026.
| Question | Professional Insight |
|---|---|
| 1. What voltage requirements should I verify before purchasing a DR chair for my clinic in 2026? | Most modern DR chairs operate on standard 110–120V (North America) or 220–240V (Europe, Asia, and other regions). However, with the increasing integration of digital imaging systems, intraoral scanners, and IoT-enabled components, ensure your chair supports stable power delivery and includes surge protection. Verify compatibility with local grid specifications and consider models with dual-voltage adaptability for multi-location clinics. Always confirm voltage and frequency (50/60 Hz) with the manufacturer prior to installation. |
| 2. How accessible are spare parts for DR chairs, and what should distributors stock? | In 2026, leading manufacturers offer globally standardized spare parts with improved traceability via QR codes and cloud-based inventory systems. Distributors should maintain stock of high-wear components such as armrests, headrests, upholstery kits, handpiece hoses, and control valves. Ensure the supplier provides a documented spare parts catalog with lead times and backward compatibility across model generations. Prioritize brands with regional distribution hubs to minimize downtime. |
| 3. What does the installation process for a DR chair involve, and do I need specialized technicians? | Installation in 2026 typically requires a certified biomedical or dental equipment technician. The process includes anchoring the chair to the floor, connecting hydraulic/pneumatic lines (if applicable), integrating with the dental unit, and calibrating digital controls and foot pedals. For chairs with embedded imaging or AI-assisted positioning, network configuration and software setup are essential. Most manufacturers offer turnkey installation packages—confirm if this is included in your purchase agreement. |
| 4. What warranty coverage is standard for DR chairs in 2026, and what does it include? | The industry standard in 2026 is a 3-year comprehensive warranty covering structural frame, motor systems, hydraulic/pneumatic components, and electronic controls. Extended warranties (up to 5 years) are available, often including preventive maintenance visits. Note: Wear items (e.g., upholstery, handpieces) may be covered under shorter terms (6–12 months). Ensure the warranty is transferable and supported locally—critical for clinic resale or relocation. |
| 5. How do I ensure long-term service support and technical updates for my DR chair? | Choose manufacturers with a dedicated clinical support network and remote diagnostic capabilities. In 2026, many DR chairs feature over-the-air (OTA) firmware updates for ergonomics, safety protocols, and compatibility with new dental tools. Confirm access to technical documentation, training modules, and responsive service hotlines. Distributors should verify service-level agreements (SLAs) for response time and part replacement within 48 hours. |
Need a Quote for Dr Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


