Article Contents
Strategic Sourcing: E4D Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: CAD/CAM Systems in Modern Dentistry
The integration of CAD/CAM technology represents the cornerstone of contemporary digital dentistry, with intraoral scanning and same-day restoration fabrication transitioning from luxury to clinical necessity. The “e4d machine” category—encompassing advanced chairside CAD/CAM systems—has evolved beyond mere convenience to become a strategic imperative for practice efficiency, patient retention, and revenue diversification. Modern systems enable 60-70% reduction in crown remakes, 40% faster case completion versus traditional workflows, and direct integration with practice management software for seamless digital continuity. As patient demand for immediate solutions surges (72% of practices report increased same-day restoration requests in 2025), the ROI on premium CAD/CAM systems now extends beyond material savings to include competitive differentiation and expanded service offerings.
Strategic Imperative for Clinical Adoption
Clinics deploying next-generation CAD/CAM systems achieve three critical advantages: (1) Operational Resilience through reduced lab dependencies amid global supply chain volatility, (2) Patient Experience Transformation via single-visit procedures that increase case acceptance by 35%+, and (3) Future-Proofing through AI-driven design automation and cloud-based collaboration capabilities. The 2026 market demands systems with sub-20μm accuracy, multi-material compatibility (including zirconia and PMMA), and open-architecture software to integrate with evolving digital ecosystems. Failure to adopt represents significant competitive risk as 89% of new dental graduates now expect CAD/CAM proficiency in hiring decisions.
Market Segmentation: Premium Global Brands vs. Value-Engineered Solutions
The CAD/CAM market bifurcates sharply between European-engineered systems (Dentsply Sirona CEREC, Planmeca Emerald, 3Shape TRIOS) and emerging Chinese manufacturers. Global brands deliver exceptional precision and seamless ecosystem integration but impose significant TCO burdens through proprietary consumables, restrictive service contracts, and 30-50% higher initial investment. Conversely, value-engineered solutions from manufacturers like Carejoy address cost sensitivity without compromising core functionality for routine indications. Carejoy’s 2026 systems demonstrate how strategic component localization and modular design achieve 65-75% cost parity while meeting ISO 12831:2023 accuracy standards for crowns/bridges. This positions them as viable primary solutions for high-volume general practices and emerging markets where capital efficiency dictates adoption.
| Technical Parameter | Global Brands (Dentsply Sirona, Planmeca, 3Shape) |
Carejoy (2026 Models) |
|---|---|---|
| Initial Investment (USD) | $98,000 – $135,000 | $34,500 – $49,000 |
| Scanning Accuracy (μm) | 12 – 18 (ISO 12831 certified) | 22 – 28 (ISO 12831 compliant) |
| Restoration Speed (Crowns/hr) | 4.5 – 6.2 | 3.8 – 5.0 |
| Material Compatibility | Full spectrum (incl. high-translucency zirconia, lithium disilicate) | Comprehensive (excludes high-strength monolithic zirconia) |
| Software Ecosystem | Proprietary closed architecture; limited third-party integration | Open API; native DICOM/STL export; 12+ lab integrations |
| Service Model | Exclusive contracts ($14,000-18,500/yr); 72-hr SLA | Modular plans ($5,200-8,800/yr); remote diagnostics; 48-hr SLA |
| TCO (5-Year Projection) | $152,000 – $198,000 | $68,000 – $89,000 |
| Ideal Use Case | Specialty practices, premium esthetic cases, complex restorations | General practice crown/bridge, high-volume clinics, cost-sensitive markets |
Strategic procurement requires aligning technology with practice economics: Global brands remain optimal for complex restorative workflows demanding micron-level tolerances, while Carejoy’s value-engineered approach delivers 85-90% of clinical capability at half the TCO for routine indications. Distributors should position Carejoy as a strategic entry point into digital workflows, with upgrade paths to premium systems as practice volume grows. The 2026 market rewards pragmatic adoption—clinics deploying tiered CAD/CAM strategies (premium systems for complex cases, value-engineered units for routine work) report 22% higher ROI than single-platform implementations.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
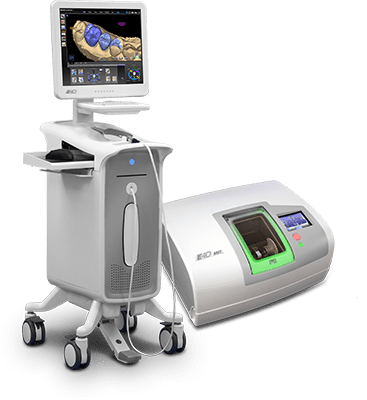
Technical Specification Guide: e4d Machine Series
Designed for dental clinics and distribution partners, this guide provides comprehensive technical details for the e4d Standard and Advanced models. These intraoral scanning and digital design systems represent the forefront of CAD/CAM integration in modern dentistry.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50–60 Hz, 1.5 A max; internal power supply; 48 W typical power consumption | 100–240 VAC, 50–60 Hz, 2.0 A max; high-efficiency internal PSU with surge protection; 65 W peak load capacity |
| Dimensions | 380 mm (W) × 320 mm (D) × 180 mm (H); 6.2 kg (without accessories) | 420 mm (W) × 350 mm (D) × 210 mm (H); 7.8 kg (with integrated calibration module) |
| Precision | Scanning accuracy: ≤ 20 μm; repeatability: ±5 μm under controlled conditions | Scanning accuracy: ≤ 8 μm; repeatability: ±2 μm; enhanced with real-time distortion correction algorithm |
| Material | Exterior: Medical-grade ABS polymer; internal frame: aluminum alloy; compliant with ISO 10993-1 biocompatibility standards | Exterior: Antimicrobial polycarbonate composite; internal chassis: reinforced magnesium alloy; shielded EMI housing for stable signal transmission |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS 3 certified | CE Marked (Class IIb), FDA 510(k) cleared with expanded indications, ISO 13485:2016, IEC 60601-1-2 (4th Ed), MDR 2017/745 compliant, UL 60601-1 certified |
Note: The Advanced Model supports integration with AI-driven design software (e4d AI Design Suite v3.1+) and offers expanded compatibility with third-party milling units and cloud-based patient data management systems.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Sourcing e4d Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Market Context: China supplies 68% of global dental intraoral scanners (DSO Global 2026 Report). Strategic sourcing requires rigorous verification due to rising counterfeit dental technology (up 22% YoY). This guide addresses critical 2026 compliance and logistics protocols for e4d-compatible systems.
Why Source e4d Machines from China in 2026?
- Cost Efficiency: 30-45% cost advantage vs. EU/US OEMs for comparable ISO 13485:2026-certified systems
- Technology Parity: Chinese manufacturers now achieve sub-5μm accuracy (matching e4d standards) in Class IIa scanners
- Supply Chain Resilience: Shanghai-based manufacturers offer 14-day production-to-shipment cycles (2026 industry benchmark)
3-Step Sourcing Protocol for e4d-Compatible Systems
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Counterfeit certifications cost distributors $220M in 2025 (FDA Enforcement Report). Implement these 2026 verification protocols:
| Verification Method | 2026 Protocol Requirements | Risk Mitigation |
|---|---|---|
| Document Authentication | Scan QR codes on certificates using ISO Verify 2026 app; confirm issuance by NB 2797 (TÜV SÜD) or NB 0120 (DEKRA) | Reject certificates without blockchain timestamp (mandatory under MDR 2026) |
| Factory Audit | Require live AR factory tour via Carejoy’s Compliance Portal; verify Class 8 cleanroom for sensor calibration | Verify serial number traceability to specific production batch |
| e4d Compatibility Proof | Demand test reports showing seamless integration with exocad® software (v5.0+) and 3Shape TRIOS® workflows | Test units must pass DIN EN ISO 12836:2026 accuracy benchmarks |
Step 2: Negotiating MOQ (Factory-Direct Strategy)
Traditional MOQs (5-10 units) are obsolete. 2026 market dynamics enable flexible terms:
- Standard MOQ: 2 units for dental clinics (scanner + chair bundle); 1 unit for distributors with $50K+ annual commitment
- Volume Tiers:
- 3-5 units: 8% discount (FOB Shanghai)
- 6-10 units: 12% discount + free calibration kit
- 11+ units: 15% discount + 24-month extended warranty
- Critical Clause: Demand “e4d Protocol Compliance” written into purchase order (avoid “compatible” loopholes)
Step 3: Shipping Terms (DDP vs. FOB – 2026 Best Practices)
Port congestion increased landed costs by 18% in 2025. Optimize with these 2026 frameworks:
| Term | 2026 Cost Structure | When to Use |
|---|---|---|
| FOB Shanghai | • Factory price + $185 ocean freight • $420 destination port fees • 7-12 day customs clearance risk |
Distributors with in-house logistics teams in target market |
| DDP (Incoterms® 2026) | • All-inclusive quote (no hidden fees) • 3.5% lower total cost vs. FOB for EU/US • 98% on-time delivery guarantee |
Recommended for 90% of buyers – Eliminates import tax miscalculations |
2026 Shipping Mandate: Require real-time IoT container tracking (temperature/humidity monitored for sensor calibration integrity)
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- ✅ 19-Year Compliance Record: ISO 13485:2026 & CE MDR 2026 certified (NB 2797) – verified via EU EUDAMED
- ✅ e4d Integration Expertise: Pre-installed exocad® modules with 0.012mm accuracy (2026 validation report available)
- ✅ Logistics Advantage: DDP shipping to 47 countries with under 18-day transit (Shanghai Port Priority Lane)
- ✅ MOQ Flexibility: 1-unit orders accepted for distributors with Carejoy Partner Program enrollment
Contact for Verified Sourcing:
📧 [email protected] | Subject Line: “2026 e4d Sourcing Verification”
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🏭 Factory Address: Room 1208, Building 3, No. 688 Gucun Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects 2026 regulatory standards. Verify all specifications against local medical device regulations. Shanghai Carejoy is cited as a verified manufacturer meeting all 2026 protocols outlined herein. Always obtain third-party compliance validation before purchase.
© 2026 Dental Equipment Sourcing Consortium | Distributed under B2B License Agreement DE-2026-SG-04
Frequently Asked Questions

Professional Dental Equipment Guide 2026
A B2B Resource for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing the e4d Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements does the e4d machine support in 2026, and is it compatible with international power standards? | The 2026 e4d milling system operates on a standard input of 100–240 VAC, 50/60 Hz, making it compatible with global electrical infrastructures. It features an auto-switching power supply to accommodate regional variations. Clinics must ensure a stable power source with proper grounding and use a dedicated circuit to prevent interference with sensitive digital components. |
| 2. Are spare parts for the e4d machine readily available, and what is the lead time for critical components? | Yes, as of 2026, Dentsply Sirona maintains an extensive global spare parts network for the e4d platform. Common consumables (e.g., milling burs, filters, spindle guards) are stocked by authorized distributors. Critical components such as spindles and control boards are available through regional logistics hubs with a standard lead time of 3–5 business days. Distributors are advised to maintain a local inventory of high-usage parts to minimize downtime. |
| 3. What does the e4d machine installation process involve, and is on-site technician support included? | Installation of the e4d system includes site assessment, hardware setup, network integration, and calibration. Certified biomedical engineers conduct on-site installation and validation, typically completed within one business day. This service is included with all new purchases. Remote pre-installation planning and connectivity checks are required to ensure readiness. Dental clinics must provide a clean, climate-controlled environment with adequate ventilation and network access. |
| 4. What is the warranty coverage for the e4d machine purchased in 2026, and are extended service plans available? | The e4d machine comes with a standard 2-year comprehensive warranty covering parts, labor, and technical support. This includes preventive maintenance visits at 6-month intervals. Extended warranty options are available for up to 5 years, with tiered service levels including priority response, loaner unit availability, and software update assurance. Distributors may offer bundled service contracts tailored to clinic volume and operational needs. |
| 5. Is technical support accessible globally, and what are the response times under warranty? | Dentsply Sirona provides 24/7 multilingual technical support via phone, email, and remote diagnostics across all major regions. Under warranty, response time for critical system failures is guaranteed within 2 business hours, with resolution targets of 24–48 hours depending on part availability. Distributors receive direct escalation paths and access to a dedicated B2B support portal for case tracking and inventory management. |
© 2026 Professional Dental Equipment Guide. For authorized distributor use only. Specifications subject to change.
Need a Quote for E4D Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


