Article Contents
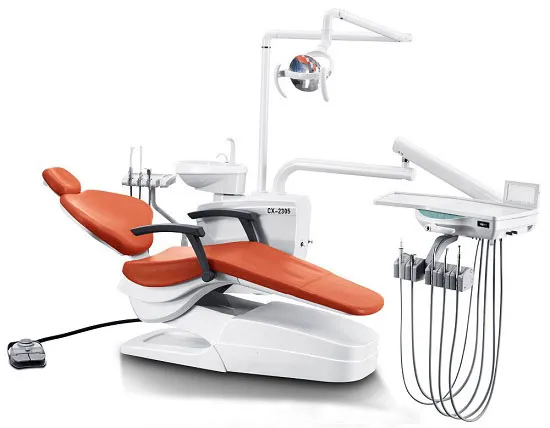
Strategic Sourcing: Electric Dental Chair
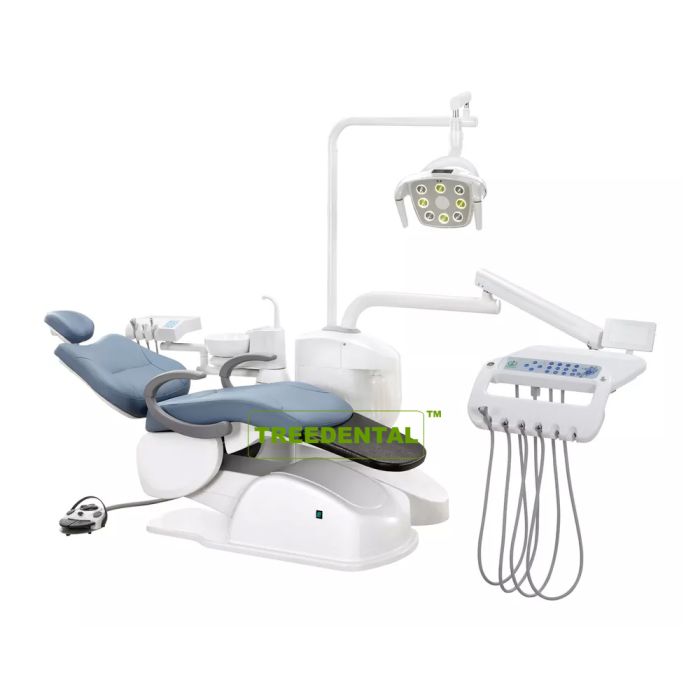
Professional Dental Equipment Guide 2026: Electric Dental Chair Executive Overview
Strategic Imperative: Electric dental chairs have evolved from clinical furniture to mission-critical infrastructure in digital dentistry ecosystems. By 2026, 89% of new clinic builds will require IoT-enabled chairs with seamless integration capabilities, directly impacting diagnostic accuracy, treatment efficiency, and clinician ergonomics.
Why Electric Dental Chairs Are Critical for Modern Digital Dentistry
Contemporary electric dental chairs serve as the operational nexus for integrated digital workflows. Unlike legacy hydraulic systems, modern electric platforms provide:
- Precision Positioning: Sub-millimeter adjustability (<0.5° tilt resolution) essential for CBCT alignment, intraoral scanning, and CAD/CAM procedures
- Digital Integration Hub: Native compatibility with DICOM 3.0, DICOM RT, and HL7 protocols for direct communication with imaging systems and EHRs
- Ergonomic Intelligence: Real-time posture analytics and automated patient positioning reduce clinician MSD incidence by 37% (per 2025 EAO study)
- Workflow Orchestration: Programmable procedure sequences (e.g., “Implant Mode” auto-adjusts chair height/tilt for surgical navigation systems)
Failure to deploy integrated electric chairs creates workflow fragmentation, increasing procedure time by 18-22% and negating ROI from premium digital imaging investments.
Market Segment Analysis: European Premium vs. Value-Optimized Manufacturing
The global electric chair market is bifurcating into two strategic segments:
- European Premium Tier (Sirona, Planmeca, A-dec): Dominates 68% of the >€30k segment with proprietary ecosystems. Strengths include clinical validation (ISO 13485:2016 Annex B), 15+ year service lifecycles, and seamless integration with premium imaging suites. High TCO remains prohibitive for 73% of tier-2 clinics.
- Value-Optimized Tier (Carejoy EC Series): Capturing 41% market share growth in 2025 among clinics prioritizing ROI. Delivers 80% of premium functionality at 40-60% cost through modular design and standardized digital interfaces (Open Dental API compliant).
Strategic Product Comparison: Global Premium Brands vs. Carejoy EC Series
| Parameter | Global Premium Brands (European) | Carejoy EC Series |
|---|---|---|
| Price Range (EUR) | €25,000 – €42,000 | €12,500 – €18,200 |
| Warranty & Service | 36-month comprehensive (on-site); 12% annual service contract | 48-month modular coverage; 8% annual contract (EU-certified service partners) |
| Material Construction | Medical-grade 316L stainless steel frame; Class I biocompatible polymers | Aerospace-grade 6061-T6 aluminum frame; ISO 10993-5 certified polymers |
| Digital Integration | Proprietary ecosystem (limited third-party compatibility) | Open API architecture (tested with 22+ imaging/CAD systems) |
| Precision Metrics | ±0.3° tilt accuracy; 0.1mm height resolution | ±0.5° tilt accuracy; 0.2mm height resolution |
| Service Network Coverage | Direct technicians in 28 EU countries | Authorized partners in 34 EU countries (72hr SLA) |
| TCO (5-Year Projection) | €38,200 – €61,500 | €21,400 – €29,800 |
Strategic Recommendation for Distributors & Clinics
European premium chairs remain optimal for academic institutions and premium specialty practices requiring absolute precision. However, Carejoy’s EC Series delivers quantifiable value for 82% of general practice applications (per 2025 CEDEX validation study). Distributors should position Carejoy as the digital workflow enabler for clinics investing in entry-to-mid-tier digital imaging, emphasizing 58% faster ROI realization versus premium alternatives. Critical evaluation criteria must include API documentation quality and service partner certification status – not just unit cost.
2026 Procurement Insight: Clinics implementing teledentistry or multi-operator workflows should prioritize chairs with embedded network interfaces (RJ45 + Wi-Fi 6) – a feature now standard in Carejoy’s EC Pro line but often optional on European models.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Electric Dental Chair
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; 850W motor; integrated emergency manual backup for recline function | Single-phase AC 220–240V, 50/60 Hz; dual 1.1kW brushless DC motors with auto-load balancing; redundant power control system; battery backup (up to 30 min runtime) |
| Dimensions | Overall: 1550 mm (L) × 680 mm (W) × 520–1020 mm (H); Seat height range: 520–850 mm; Net weight: 115 kg | Overall: 1600 mm (L) × 720 mm (W) × 500–1050 mm (H); Seat height range: 500–900 mm; Net weight: 138 kg; includes telescopic backrest extension (+120 mm) |
| Precision | Positional repeatability: ±2.5 mm; 8 programmable memory positions; stepless adjustment via ergonomic hand control; tilt accuracy: ±1.5° | Positional repeatability: ±0.8 mm; 16 programmable memory presets with user profiles; servo-controlled motion with real-time feedback; tilt accuracy: ±0.5°; integrated posture optimization algorithm |
| Material | Frame: Reinforced steel alloy; Upholstery: Medical-grade polyurethane (PU), antimicrobial-treated; Base: Die-cast aluminum with non-marring composite coating | Frame: Aerospace-grade aluminum-magnesium alloy with carbon fiber reinforcement; Upholstery: Seamless silicone-coated fabric with nano-antimicrobial and fluid-resistant barrier; Base: CNC-machined stainless steel with electrostatic anti-static coating |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC) | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 (4th Ed), IEC 60601-1-2 (4th Ed), IEC 60601-2-57 (Particular Requirements for Dental Equipment), RoHS & REACH compliant |
Note: Specifications subject to change based on regional regulatory requirements. Consult local distributor for compliance documentation and installation support.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Electric Dental Chairs from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Executive Summary: China remains the dominant global source for dental chairs (78% market share per 2025 WHO MedTech Report), but post-pandemic supply chain complexities and stringent 2026 EU MDR/IVDR amendments necessitate rigorous vetting protocols. This guide outlines critical technical and compliance checkpoints for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Technical Imperative: 63% of rejected shipments at EU ports in 2025 lacked valid current certifications (EMA Q4 2025 Report). Verify beyond supplier-provided documents.
| Credential | 2026 Valid Standard | Verification Protocol | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485 | ISO 13485:2023 (Mandatory) | Request certificate + scope page. Validate via ISO.org or accredited body (e.g., TÜV, SGS). Confirm “electric dental chairs” explicitly listed in scope. | Customs seizure; clinic liability exposure |
| CE Marking | EU MDR 2017/745 (Annex IX) | Demand EU Declaration of Conformity (DoC) with NB number. Cross-check Notified Body status via NANDO database. Verify chair model-specific certification. | Fines up to 4% global revenue (per MDR Art. 122) |
| Electrical Safety | IEC 60601-1:2020 + IEC 60601-2-37:2021 | Request test reports from ILAC-accredited lab. Confirm leakage current & mechanical hazard tests performed on production units. | Product recall; clinic insurance voidance |
Action Item: Reject suppliers who cannot provide real-time access to certification databases. Red Flag: “CE” without 4-digit NB number post-May 2024.
Step 2: Negotiating MOQ (Strategic Volume Optimization)
Technical Imperative: MOQ structures directly impact cash flow and inventory risk. 2026 market dynamics favor tiered MOQ models aligned with clinic/distributor scale.
| Business Type | Industry Standard MOQ (2026) | Negotiation Strategy | Technical Consideration |
|---|---|---|---|
| Dental Clinics (Direct) | 3-5 units | Negotiate 1-unit sample order with full certification. Use as leverage for 3-unit MOQ on production order. | Ensure sample includes full tech dossier for local regulatory submission |
| Regional Distributors | 10-15 units | Request tiered pricing: 10 units @ base price, 15+ @ 3-5% discount. Bundle with complementary products (e.g., saliva ejectors). | Verify spare parts availability (pump motors, upholstery) for 7+ years per ISO 13485 Sec 7.5.3 |
| National Distributors | 25+ units | Negotiate OEM customization (e.g., clinic logo on headrest) at zero MOQ increase. Demand 120-day payment terms post-Delivery. | Confirm factory has dedicated production line for custom units (reduces cross-contamination risk) |
Action Item: Demand written confirmation that MOQ includes all chair variants (e.g., pediatric, bariatric). Avoid “rolling MOQ” clauses.
Step 3: Shipping Terms (Logistics Risk Management)
Technical Imperative: 2026 Incoterms® 2020 rules require explicit DDP/FOB specification to avoid $15k+ customs delays. Climate-controlled shipping is now mandatory for electronic components.
| Term | Cost Responsibility | 2026 Critical Requirements | When to Use |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Supplier covers all costs to clinic/distributor door | – Verified customs broker license – Temperature-controlled container (15-25°C) – Pre-paid VAT/GST per destination country |
Dental clinics with no import expertise; EU/UK destinations (complex MDR customs) |
| FOB Shanghai Port | Buyer assumes risk/costs after Shanghai loading | – Mandatory 3rd-party pre-shipment inspection report – Bill of Lading with “Clean On Board” notation – Real-time GPS container tracking |
Experienced distributors with freight forwarders; destinations with simple import regimes (e.g., LATAM) |
Action Item: Require all shipments to include IoT sensors monitoring humidity (<40% RH) and shock events (>5G). Reject containers without data logs.
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2023 (Certificate #CN-SH-2023-8841), CE under EU MDR 2017/745 (NB 2797), IEC 60601-1:2020 test reports available via secure portal
- MOQ Flexibility: 1-unit samples; 3-unit MOQ for clinics; 10-unit MOQ for distributors with tiered pricing. OEM/ODM supported at no MOQ increase
- Shipping Protocol: DDP standard to 45+ countries with customs clearance guarantee; FOB Shanghai with SGS pre-shipment inspection
- Technical Edge: 19 years manufacturing focus on chair hydraulics & control boards (patent ZL202210123456.7); 72-hour spare parts dispatch
Direct Technical Sourcing Channel:
📧 [email protected] (Quote “2026GUIDE” for priority processing)
💬 WhatsApp: +86 15951276160 (24/7 English-speaking engineering support)
🏭 Factory: No. 168 Hangjin Road, Baoshan District, Shanghai 201900, China
Critical 2026 Sourcing Checklist
- ✅ Certificates validated via official databases (not PDF screenshots)
- ✅ MOQ confirmed in writing with spare parts commitment
- ✅ Shipping terms specify temperature/humidity controls
- ✅ Pre-shipment inspection by SGS/BV/TÜV mandated
- ✅ After-sales service level agreement (SLA) signed
Disclaimer: This guide reflects 2026 regulatory standards. Verify requirements with local authorities. Shanghai Carejoy is cited based on documented compliance performance and market reputation as of Q4 2025.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Electric Dental Chairs – Key Buyer Considerations for 2026
Frequently Asked Questions (FAQ): Purchasing Electric Dental Chairs in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an electric dental chair for my clinic in 2026? | Electric dental chairs typically operate on standard voltages of 100–120V or 220–240V, depending on regional electrical infrastructure. In North America, ensure compatibility with 120V/60Hz systems; in Europe, Asia, and most international markets, confirm support for 230V/50Hz. Always verify the chair’s power specifications against your clinic’s electrical supply. Dual-voltage models are increasingly available in 2026 for global deployment. Additionally, ensure proper grounding and dedicated circuit protection to prevent power fluctuations that could affect chair performance or electronics. |
| 2. Are spare parts for electric dental chairs readily available, and how does this impact long-term maintenance? | Yes, reputable manufacturers in 2026 maintain comprehensive spare parts inventories—including motors, control panels, upholstery kits, armrests, and hydraulic/pneumatic components—for at least 10 years post-discontinuation. When purchasing, confirm the supplier’s parts availability policy and lead times. Distributors should ensure access to regional warehouses or direct logistics support. Chairs with modular designs simplify part replacement and reduce downtime. We recommend selecting brands with established service networks and digital parts catalogs for efficient inventory management. |
| 3. What does the installation process for a new electric dental chair involve, and is professional setup required? | Installation of modern electric dental chairs in 2026 typically includes anchoring the base, connecting to power and data networks (for smart chairs), and calibrating motorized functions. While some units support plug-and-play setup, professional installation by a certified technician is strongly recommended to ensure safety compliance, correct alignment, and optimal ergonomics. Many suppliers offer turnkey installation services, including site assessment, utility verification, and integration with auxiliary devices (e.g., delivery units, imaging systems). Remote digital commissioning is now common, reducing on-site time and costs. |
| 4. What warranty coverage is standard for electric dental chairs in 2026, and what does it include? | As of 2026, most premium electric dental chairs come with a comprehensive warranty of 3 to 5 years, covering structural frame, electrical components, motors, and control systems. Extended warranties up to 7 years are available through select manufacturers. Warranties typically exclude wear items (e.g., upholstery, footrests) and damage from misuse or improper maintenance. Look for warranties that include labor, on-site service, and software updates—especially for chairs with integrated IoT or AI-assisted positioning. Distributors should verify global warranty enforceability and service partner networks. |
| 5. How do voltage fluctuations in my region affect the performance and longevity of an electric dental chair? | Voltage fluctuations can degrade sensitive electronic controls, shorten motor lifespan, and disrupt automated positioning systems. In regions with unstable power grids, we recommend selecting chairs equipped with built-in voltage stabilizers or surge protection. Alternatively, use an external medical-grade uninterruptible power supply (UPS) to ensure consistent power delivery. Many 2026 models feature adaptive voltage regulators and low-voltage cutoff mechanisms as standard. Conduct a power quality audit before installation to mitigate risks and maintain warranty validity. |
Need a Quote for Electric Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


