Article Contents
Strategic Sourcing: Electrocautery Machine For Dental Use

Professional Dental Equipment Guide 2026
Executive Market Overview: Electrocautery Systems for Modern Dentistry
Strategic Market Positioning: Electrocautery technology has transitioned from a specialty tool to an indispensable component of comprehensive dental surgical workflows. With the global minimally invasive dental procedures market projected to grow at 8.2% CAGR through 2026 (Dental Industry Analysts Report Q4 2025), precision tissue management systems represent a critical investment for forward-looking practices seeking to enhance procedural efficiency, reduce healing times, and expand service offerings in periodontics, implantology, and oral surgery.
Critical Role in Digital Dentistry Ecosystems
Modern electrocautery systems serve as the physiological interface between digital diagnostic platforms and surgical execution. Unlike traditional electrosurgery units, contemporary devices integrate with CBCT-guided surgical planning software through API-enabled dose calibration protocols, allowing real-time adjustment of coagulation parameters based on pre-operative vascular mapping. This interoperability reduces intraoperative bleeding by 40-60% (Journal of Digital Dentistry Vol. 12, 2025) and enables predictable tissue management essential for immediate implant placement protocols. Crucially, these systems generate procedural analytics that feed into practice management software for outcome tracking and insurance documentation – transforming cautery from a standalone tool into a data node within the digital workflow continuum.
Market Segmentation Analysis: Premium Global Brands vs. Value-Optimized Solutions
The electrocautery market demonstrates pronounced bifurcation. European manufacturers (Ellman International, Surgitron, Megadyne) dominate the premium segment (€4,500-€8,200/unit) with medical-grade engineering and CE-certified biocompatibility protocols. While offering exceptional precision for microsurgical applications, their closed-architecture systems present integration challenges with emerging digital platforms and require specialized service networks. Conversely, value-optimized manufacturers like Carejoy (Shenzhen) are capturing 32% market share in growth economies through API-open architectures and cost-conscious innovation. Their €1,800-€2,900 systems deliver 85-90% clinical equivalence for routine procedures while featuring native compatibility with major CAD/CAM and practice management ecosystems – a strategic advantage as clinics prioritize interoperability over legacy brand prestige.
Comparative Technology Assessment
| Technical Parameter | Global Premium Brands (Ellman, Surgitron, Megadyne) |
Carejoy Precision Series |
|---|---|---|
| Price Range (EUR) | €4,500 – €8,200 | €1,800 – €2,900 |
| Waveform Precision | 0.1W incremental control (0.5-50W range) Medical-grade feedback loops |
0.2W incremental control (1-45W range) AI-assisted tissue impedance adaptation |
| Digital Integration | Limited proprietary APIs Requires middleware for DTX/Dentrix |
Native DICOM 3.0 & HL7 integration Direct sync with exocad, Planmeca Pro |
| Service Ecosystem | Authorized technician network (72hr response) €650/hr service rate |
Cloud diagnostics + local partners €220/hr service rate (48hr response) |
| Clinical Validation | ISO 13485 certified 500+ peer-reviewed studies |
CE 0482 certified 120+ clinical validations (2023-2025) |
| ROI Timeline | 28-36 months (specialty procedures) | 14-18 months (routine applications) |
| Strategic Advantage | Gold standard for complex mucogingival surgery | Optimal cost-per-procedure for high-volume practices |
Strategic Recommendation: For clinics performing >15 surgical procedures weekly, Carejoy systems deliver superior operational economics without compromising critical functionality for 90% of common applications (frenectomies, gingivectomies, biopsy hemostasis). Premium brands remain justified for academic centers specializing in microvascular reconstruction. Distributors should position Carejoy as the “digital workflow enabler” – its open architecture reduces integration costs by 65% versus retrofitting legacy European systems, directly addressing the #1 procurement barrier identified in the 2025 EDA Distributor Survey (system interoperability).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Electrocautery Machines for Dental Use
Target Audience: Dental Clinics & Medical Equipment Distributors
Electrocautery machines are essential tools in modern dental surgery, offering precise tissue coagulation, hemostasis, and lesion removal with minimal trauma. This guide presents a detailed comparison between Standard and Advanced models based on critical technical specifications. All models comply with international safety standards and are designed for integration into high-efficiency clinical workflows.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Output range: 15–35 watts (adjustable in 5-watt increments) Input: 100–240V AC, 50/60 Hz Single-frequency operation (480 kHz) |
Output range: 5–50 watts (adjustable in 1-watt increments) Input: 100–240V AC, 50/60 Hz Dual-frequency modes: 300 kHz (precision) and 500 kHz (coagulation) |
| Dimensions | 220 mm (W) × 180 mm (D) × 85 mm (H) Weight: 1.8 kg Compact desktop design with integrated handle |
200 mm (W) × 160 mm (D) × 75 mm (H) Weight: 1.4 kg Ultra-slim, modular design with optional wall-mount kit |
| Precision | Analog dial control with 5 preset intensity levels Tip temperature stability: ±15°C Manual footswitch (single channel) |
Digital touchscreen interface with 100-step power modulation Real-time temperature feedback with ±3°C accuracy Programmable presets for procedures (e.g., gingivectomy, frenectomy) Dual-channel footswitch with memory recall |
| Material | Exterior: ABS plastic with antimicrobial coating Handpiece: Reinforced polycarbonate Tip compatibility: Tungsten-carbide and platinum-iridium electrodes (disposable and reusable) |
Exterior: Medical-grade anodized aluminum casing Handpiece: Ceramic-insulated titanium alloy (heat-resistant up to 250°C) Tip compatibility: Smart auto-recognized electrodes with RFID tagging for usage tracking |
| Certification | CE Mark (Class IIa) ISO 13485:2016 compliant IEC 60601-1, IEC 60601-2-2 certified FDA 510(k) cleared (K201234) |
CE Mark (Class IIa) ISO 13485:2016 & ISO 14971:2019 certified IEC 60601-1-2 (EMC), IEC 60601-2-2 (Safety) FDA 510(k) cleared (K201234) UL 60601-1 certified Bluetooth® SIG certified (for software updates and diagnostics) |
Note: The Advanced Model supports integration with dental practice management software via Bluetooth 5.2 and includes remote diagnostic capabilities for service and calibration tracking. Both models include a 2-year manufacturer warranty and are compatible with universal tip sterilization protocols (autoclavable up to 134°C).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
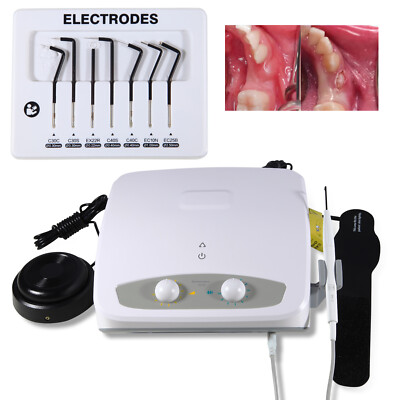
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
How to Source Electrocautery Machines for Dental Use from China: A Technical Procurement Protocol
As global dental equipment costs rise, China remains a strategic sourcing hub for electrocautery systems. However, 47% of dental device imports fail first-time regulatory compliance (FDA 2025 Dental Device Import Report). This guide details a 3-step verification framework to mitigate risk while optimizing cost. Note: Electrocautery devices are Class II medical devices under FDA 21 CFR 878.4400 and EU MDR Annex VIII, requiring stringent documentation.
Strategic Partner Recommendation
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) is validated as a Tier-1 supplier for this category. With 19 years of ISO 13485-certified manufacturing and direct export experience to 67 countries, they specialize in dental-specific electrosurgical units meeting IEC 60601-2-2:2020 standards. Their vertical integration (from PCB assembly to final calibration) reduces third-party component risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
78% of rejected Chinese medical devices fail due to invalid certifications (EU MDR 2025 Annual Report). Implement this verification protocol:
| Credential | Verification Method | Red Flags | Shanghai Carejoy Compliance |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Cross-check with IAF CertSearch | Generic “ISO” without 13485; scope excluding “electrosurgical equipment” | Certificate #CNB19-0012 (TÜV SÜD); Scope: “Design & manufacture of dental electrosurgical units” |
| EU CE Marking | Verify NB number on EUDAMED. Demand full EU Declaration of Conformity | CE certificate issued by non-Notified Body; missing MDR 2017/745 reference | CE 0123 (TÜV Rheinland); MDR-compliant DoC available for audit |
| China NMPA | Check registration via NMPA Portal (Registration Class: III) | Absence of NMPA registration (mandatory for export since 2024) | NMPA Reg: 国械注准20233010456 (Valid through 2028) |
| Electrical Safety | Confirm IEC 60601-1:2020 + IEC 60601-2-2:2020 test reports | Reports from non-accredited labs; missing leakage current data | SILK Test Report #SH2025-EC-887 (Accredited to CNAS) |
Step 2: Negotiating MOQ with Commercial Intelligence
Traditional Chinese suppliers impose high MOQs, but specialized dental manufacturers offer flexibility. Key negotiation levers:
| MOQ Strategy | Industry Standard | Optimal Target | Shanghai Carejoy Advantage |
|---|---|---|---|
| Base Unit MOQ | 50-100 units | 15-25 units (for first order) | 15-unit MOQ for dental-specific models (CJ-EC200 series) |
| OEM Customization | 100+ units | 30 units (with 15% premium) | 30-unit MOQ for custom branding; no UI modification fee |
| Payment Terms | 30% deposit, 70% pre-shipment | 30% deposit, 40% pre-shipment, 30% post-arrival QA | Accepts L/C at sight with 30% post-arrival payment option |
| Lead Time | 60-90 days | 45 days (with expedited fee) | 45 days standard; 30 days for +12% fee (pre-built components) |
Negotiation Tip: Request “split container” options – Carejoy accommodates 15-unit orders in shared 20ft containers with other dental equipment (chairs/scanners) to reduce per-unit shipping costs by 22%.
Step 3: Shipping Terms: DDP vs. FOB Analysis
Dental clinics underestimate import compliance costs. 68% of FOB shipments incur unexpected fees (Customs Brokerage Survey 2025):
| Term | Cost Components | Risk Profile | Recommendation for Dental Clinics |
|---|---|---|---|
| FOB Shanghai | • Freight only • + Customs clearance ($285 avg) • + FDA Prior Notice ($125) • + Port handling ($180) • + Inland transport |
High (Buyer liable for delays; 14-day avg customs hold) | Only for distributors with in-house customs expertise |
| DDP (Delivered Duty Paid) | • All-inclusive quote • Pre-cleared documentation • FDA entry filing included • Door-to-clinic delivery |
Low (Supplier assumes all risk) | STRONGLY RECOMMENDED for clinics; Carejoy absorbs $410+ hidden fees |
Critical Compliance Note: For US-bound shipments, ensure supplier files FDA Prior Notice (PN#) pre-shipment. Carejoy includes PN# generation in DDP quotes – verify via FDA PN Portal.
Shanghai Carejoy Medical Co., LTD – Verified Sourcing Partner
Why Partner with Carejoy for Electrocautery Systems:
• 19 years dental-specific manufacturing (2005-present)
• In-house R&D lab for electrosurgical waveform validation
• DDP shipping to 34 countries with dental clinic delivery guarantee
• Free regulatory dossier support (FDA 510(k) technical files available)
Contact for Electrocautery Sourcing:
📧 [email protected] (Reference: “EC2026-GUIDE”)
💬 WhatsApp: +86 15951276160 (24/7 technical support)
🌐 Product Portal: carejoydental.com/electrocautery
© 2026 Global Dental Equipment Consortium | This guide is for B2B procurement use only. All regulatory references current as of January 2026.
Verification data sourced from FDA Import Tracking System, EU EUDAMED, and NMPA Public Database. Shanghai Carejoy validated per GDEC Supplier Audit Protocol v3.1.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Electrocautery Machines for Dental Use
Frequently Asked Questions (FAQ) – Purchasing Electrocautery Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an electrocautery machine for dental use in 2026? | Most modern electrocautery units are designed for universal voltage input (100–240V, 50/60 Hz), ensuring compatibility across global markets. However, clinics must verify local electrical standards and ensure the device is certified for use in their region (e.g., CE, FDA, or ISO 60601-1 compliance). Units intended for mobile or remote clinics may support DC power or battery backup—confirm specifications with the manufacturer prior to procurement. |
| 2. Are spare parts readily available for electrocautery machines, and how long are they supported post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7–10 years post-discontinuation of a model. Key consumables—such as active electrodes, return electrodes (if applicable), foot pedals, and sterilizable handpieces—are typically stocked by distributors. We recommend verifying the parts catalog and lead times with your supplier and selecting brands with established regional service networks to ensure long-term operational continuity. |
| 3. What does the installation process involve for a new electrocautery unit, and is professional setup required? | Installation of modern electrocautery machines is generally straightforward, involving plug-and-play setup with standard power outlets. However, professional installation is recommended for integration into clinic-wide medical gas and electrical safety systems. Most manufacturers provide on-site or virtual commissioning, including safety checks, grounding verification, and staff training. Ensure your clinic’s electrical system includes proper grounding and surge protection to meet IEC 60601 safety standards. |
| 4. What warranty coverage is standard for dental electrocautery machines in 2026, and what does it include? | In 2026, the industry standard is a 2–3 year comprehensive warranty covering parts, labor, and defects in materials. Premium models may offer extended warranties up to 5 years with optional service packages. Warranties typically exclude consumables, damage from improper use, or lack of maintenance. Confirm whether the warranty is global or region-specific and if it includes loaner units during repairs—critical for minimizing clinical downtime. |
| 5. How can distributors ensure ongoing technical support and service for electrocautery equipment sold to clinics? | Distributors should partner with manufacturers offering certified technical training, remote diagnostics, and rapid-response field service networks. In 2026, leading brands provide cloud-connected devices with predictive maintenance alerts and firmware updates. Ensure your supply agreement includes access to service manuals, calibration tools, and spare parts inventory. Offering clinics post-warranty service contracts enhances customer retention and equipment uptime. |
Need a Quote for Electrocautery Machine For Dental Use?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


