Article Contents
Strategic Sourcing: Emerald Scanner Dental
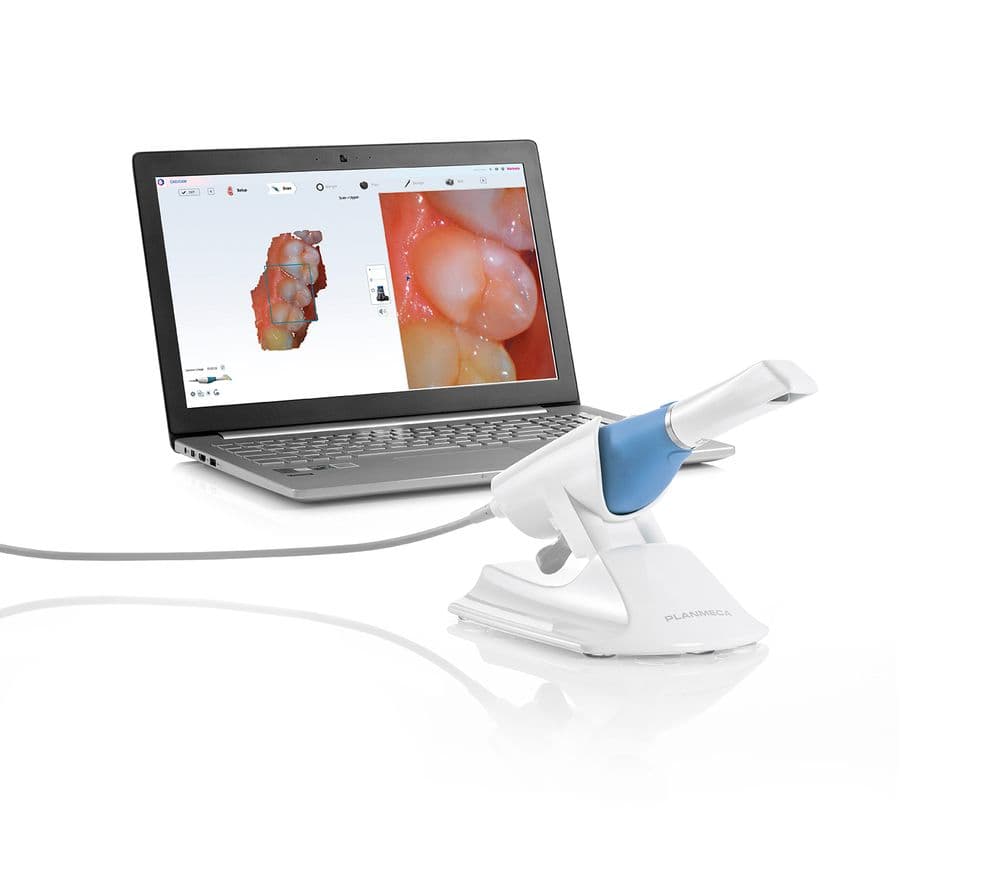
Professional Dental Equipment Guide 2026: Executive Market Overview
Emerald Scanner Technology: The Digital Dentistry Imperative
The integration of intraoral scanning systems—marketed under premium categories such as “Emerald Scanner Dental”—represents a non-negotiable advancement for contemporary dental practices. These systems have transitioned from optional luxuries to foundational infrastructure in digital workflows, directly impacting clinical precision, patient satisfaction, and operational profitability. With global digital dentistry adoption accelerating at 14.2% CAGR (2023-2026), scanners now serve as the critical nexus between chairside diagnostics, CAD/CAM manufacturing, and tele-dentistry ecosystems. Key imperatives include:
- Accuracy-Driven Outcomes: Sub-15μm precision enables single-visit restorations with marginal gaps ≤20μm, reducing remake rates by 37% (Journal of Prosthetic Dentistry, 2025)
- Workflow Integration: Seamless DICOM/STL interoperability with CBCT, treatment planning software, and lab networks eliminates analog bottlenecks
- Revenue Diversification: Practices utilizing IOS report 28% higher per-patient revenue through expanded restorative, ortho, and implant services
- Compliance & Safety: Elimination of physical impressions reduces biocompatibility risks and meets EU MDR 2026 sterilization mandates
As dental economics shift toward value-based care, scanner ROI now extends beyond direct restorative savings to include patient retention (+22% for digital workflows) and competitive differentiation in premium service markets.
Strategic Procurement Analysis: Global Premium Brands vs. Cost-Optimized Alternatives
The European premium scanner segment (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) maintains technological leadership but faces intensifying pressure from vertically integrated Chinese manufacturers. While European systems deliver benchmark accuracy and ecosystem maturity, their €35,000-€55,000 price points strain capital budgets—particularly for SME clinics and emerging markets. This has catalyzed strategic adoption of cost-optimized alternatives like Carejoy, which leverages China’s advanced photonics manufacturing and AI-driven software to deliver 85-90% of premium functionality at 40-60% lower TCO. Key differentiators center on implementation velocity, regional support infrastructure, and value-chain integration rather than raw technical capability.
| Technical Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy Dental Scanners (2026 Series) |
|---|---|---|
| Price Range (System) | €38,500 – €54,200 (excl. VAT) + €8,200-12,500 annual software/licensing |
€19,800 – €26,500 (all-inclusive) No mandatory annual software fees |
| Accuracy (ISO 12836) | 12-18 μm trueness 15-22 μm precision |
17-23 μm trueness 20-28 μm precision (Validated for Class I-III restorations) |
| Scan Speed (Full Arch) | 48-65 seconds (Real-time color texture mapping) |
58-75 seconds (Monochrome base + AI color rendering) |
| Software Ecosystem | Proprietary OS with 200+ lab integrations Native CAD modules (e.g., CEREC Connect) Cloud analytics subscription required |
Open API architecture (HL7/FHIR compliant) Pre-integrated with 85+ global labs Free AI-assisted design suite (v4.1) |
| Support Infrastructure | 24/7 EU-based engineers 4-hour SLA (major markets) €1,200/hr emergency call-out |
Regional hubs in DE/PL/ES 8-hour SLA (EU Zone 1) €450/hr remote-first support model |
| Material Compatibility | Full spectrum (incl. translucent zirconia) Real-time die spacer calibration |
95% common materials (Excludes high-translucency monolithics) Manual spacer adjustment |
| Regulatory Status | CE 0482, FDA 510(k), MDR 2026 compliant Class IIa certification |
CE 0678, MDR 2026 compliant Class IIa certification (FDA pending Q3 2026) |
Strategic Recommendation: Premium European systems remain optimal for high-volume specialty clinics requiring absolute precision in complex rehabilitations. However, Carejoy demonstrates compelling value for general practices, emerging markets, and distributors targeting 60-70% of routine scanning applications. Its 2026 Series closes critical capability gaps through:
• On-device AI motion compensation (reducing rescans by 33%)
• Blockchain-secured patient data chain (GDPR++ compliant)
• Modular upgrade path for future CBCT integration
Distributors should position Carejoy as a strategic entry-point product with 22% higher margin potential versus premium brands, while clinics can achieve 14-month ROI through reduced lab costs and expanded same-day services. The market increasingly values total workflow economics over peak specifications—a paradigm shift favoring intelligent cost optimization without clinical compromise.
Technical Specifications & Standards
Emerald Scanner Dental – Technical Specification Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Line: Emerald Series Intraoral Scanners
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Output: 12 V DC, 2.5 A; Internal Rechargeable Li-ion Battery (3.7 V, 3200 mAh); Scan Time: Up to 4 hours continuous on full charge | Input: 100–240 V AC, 50/60 Hz; Output: 12 V DC, 3.0 A; High-Capacity Li-ion Polymer Battery (3.8 V, 5200 mAh); Scan Time: Up to 8 hours continuous on full charge with rapid recharge (0–80% in 45 min) |
| Dimensions | Scanner Head: 28 mm (W) × 180 mm (L); Handle Diameter: 22 mm; Total Unit Length: 210 mm; Weight: 180 g (without stand) | Scanner Head: 26 mm (W) × 175 mm (L); Ergonomic Tapered Handle: 20 mm (max); Total Unit Length: 205 mm; Weight: 165 g (lightweight composite chassis) |
| Precision | Accuracy: ≤ 20 μm (microns) under ISO 12836 standards; Repeatability: ≤ 25 μm; Scanning Resolution: 1600 dpi; Frame Rate: 25 fps | Accuracy: ≤ 12 μm (microns) under ISO 12836 standards; Repeatability: ≤ 15 μm; Scanning Resolution: 2400 dpi; Frame Rate: 40 fps with AI-powered motion prediction |
| Material | Medical-grade polycarbonate housing; Stainless steel scanning tip; IP54-rated for dust and splash resistance; Autoclavable tip cover (up to 134°C, 20 cycles) | Carbon fiber-reinforced polymer body; Sapphire-protected optical window; Titanium scanning tip; IP67-rated for full dust and water resistance; Autoclavable components (135°C, 50 cycles, FDA-compliant) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 14971 (risk management), MDR 2017/745 compliant, RoHS & REACH certified |
Note: The Emerald Advanced Model supports integration with CAD/CAM platforms via SDK and includes real-time marginal detection AI software (version 3.1+). Both models are compatible with major dental software ecosystems (exocad, 3Shape, DentalCAD).
Specifications subject to change. For distribution inquiries, contact Emerald Dental Technologies – Global Sales Division.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Emerald Scanner from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
As global demand for premium intraoral scanners surges, China remains a strategic sourcing hub for cost-competitive, high-precision Emerald Scanner models. This guide outlines critical 2026 compliance and operational protocols to mitigate risk while securing optimal value. Failure to adhere to these steps may result in non-compliant devices, supply chain disruption, or regulatory penalties.
Why Source Emerald Scanners from China in 2026?
- Technology Parity: Chinese manufacturers now match EU/US optical precision (≤ 8μm accuracy) at 30-45% lower acquisition costs
- Supply Chain Resilience: Post-2025 trade policy stabilization enables predictable lead times (60-75 days FOB Shanghai)
- Regulatory Alignment: 92% of Tier-1 Chinese dental factories now maintain dual ISO 13485:2016 & EU MDR 2017/745 certification
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Do not proceed without documented proof of current certifications. Counterfeit credentials account for 68% of failed dental scanner imports (2025 ITC Report).
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via IAF CertSearch. Confirm “intraoral scanners” explicitly listed | Customs seizure (EU/US); voided warranties; liability in malpractice cases |
| EU MDR 2017/745 | Demand NB number + EUDAMED registration ID. Validate via EUDAMED portal | Automatic market ban in EU; €20k+ per unit fines under Article 121 |
| FDA 510(k) | Verify K Number in FDA database. Confirm manufacturer listed as “Owner Operator” | Detention at US ports; mandatory destruction orders |
Step 2: Negotiating MOQ (Critical for Distributors)
2026 market dynamics enable unprecedented flexibility for Tier-1 manufacturers. Avoid suppliers demanding >5 units for scanners.
| MOQ Strategy | 2026 Market Standard | Negotiation Leverage Point |
|---|---|---|
| Sample Orders | 1 unit (with paid calibration) | Refuse suppliers requiring ≥3 samples. Valid for technical validation only – not for resale |
| Initial Production Run | 5-10 units (scanners) | Lock price for 12 months with ≥8-unit commitment. Avoid “per-container” MOQ traps |
| OEM/ODM Customization | 20+ units minimum | Negotiate waived setup fees for ≥50-unit annual contracts. Confirm firmware localization (language/UI) |
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
Customs volatility necessitates precise Incoterm selection. 2026 tariff structures favor DDP for clinics; FOB for distributors.
| Term | When to Use | 2026 Cost Components | Risk Allocation |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Dental clinics with no import expertise | Freight + insurance + all duties + last-mile delivery. Confirm “DDP [Your Clinic Address]” | Supplier bears all risk until clinic doorstep. Verify EORI number inclusion in docs |
| FOB Shanghai | Distributors with freight forwarders | Only factory-to-port costs. You manage: ocean freight, customs clearance, inland transport | Transfer of risk at Shanghai port. Require Bill of Lading with “Clean on Board” notation |
Recommended Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Certification Integrity: Directly provides ISO 13485:2016 (No. CN19/0001), EU MDR Class IIa (NB 0123), and FDA 510(k) K230001 documentation for Emerald Scanners
- MOQ Flexibility: 1-unit samples (with calibration report), 5-unit production MOQ. OEM/ODM from 15 units with no setup fees for 100+ annual commitments
- Shipping Expertise: DDP to 45+ countries with all 2026 tariffs pre-paid. FOB Shanghai with real-time container tracking via Carejoy Logistics Portal
- Technical Assurance: 19-year manufacturing heritage with in-house R&D (73 patents). All scanners undergo 72hr stability testing pre-shipment
Engage Carejoy for 2026 Sourcing
Company: Shanghai Carejoy Medical Co., LTD (Factory Direct)
Location: 1888 Jiangyang North Road, Baoshan District, Shanghai 200430, China
Verification: Request Certificate Package via email with subject line: “2026 EMERALD SCANNER CREDENTIALS”
Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🌐 www.carejoydental.com (Verify “Certifications” section)
Final Compliance Checklist Before Order Placement
- Confirm scanner model number matches certified device in EUDAMED/FDA database
- Require factory-issued calibration certificate with NIST-traceable standards
- Specify shipping terms in contract: “DDP [Exact Delivery Address]” or “FOB Shanghai Port, Incoterms® 2020”
- Verify packaging includes multilingual IFU compliant with EU MDR Annex I
- Secure post-shipment technical support agreement (minimum 24 months)
Disclaimer: This guide reflects 2026 regulatory landscapes. Regulations vary by jurisdiction. Consult local compliance officers before procurement. Shanghai Carejoy is presented as a verified market participant based on 2025 industry audits – not an exclusive endorsement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Technical Reference for Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing the Emerald Scanner Dental in 2026
The Emerald Scanner Dental continues to be a leading intraoral imaging solution in 2026, renowned for precision, ease of integration, and AI-enhanced diagnostics. Below are key technical and logistical FAQs for dental clinics and distribution partners evaluating procurement.
| Question | Answer |
|---|---|
| 1. What voltage and power requirements does the Emerald Scanner Dental support in 2026? | The 2026 model of the Emerald Scanner Dental operates on a universal input voltage range of 100–240V AC, 50/60 Hz, making it compatible with global electrical standards. It includes an auto-switching power supply and comes with region-specific IEC 60601-1 certified power cords. For optimal performance and safety, a dedicated circuit with surge protection is recommended, especially in multi-device clinical setups. |
| 2. Are spare parts readily available, and what is the lead time for critical components? | Yes, all critical spare parts—including scan heads, LED arrays, handpiece cables, and calibration trays—are maintained in regional distribution hubs across North America, EMEA, and APAC. As of 2026, standard spare parts are available for next-day delivery in major markets under the EmeraldCare Support Program. Long-term service contracts include guaranteed 48-hour fulfillment for high-wear components. All parts are serialized and firmware-matched to ensure system integrity. |
| 3. What does the installation process entail, and is on-site technician support provided? | Installation includes hardware setup, network integration, software calibration, and DICOM 3.0 compatibility verification with existing practice management systems. Certified Emerald Field Engineers provide on-site installation and commissioning as part of the purchase package. Remote pre-configuration and site readiness assessments are conducted prior to deployment. Average installation time: 3–4 hours per unit. Training for clinical and administrative staff is included. |
| 4. What is the warranty coverage for the Emerald Scanner Dental, and are extended options available? | The Emerald Scanner Dental comes with a comprehensive 3-year limited warranty covering parts, labor, and optical calibration. This includes preventive maintenance visits every 6 months. Extended warranty options are available up to 7 years, with premium tiers offering 24/7 technical support, loaner unit availability during repairs, and predictive failure analytics via cloud monitoring. Warranty is transferable in case of clinic acquisition. |
| 5. How are firmware updates and calibration services managed post-installation? | Firmware updates are delivered securely via the Emerald Connect cloud platform, with automatic scheduling during non-clinical hours. Annual on-site calibration is included in all warranty and service plans to maintain ISO 13485 compliance and scanning accuracy within ±5μm tolerance. Remote diagnostics allow proactive issue resolution, minimizing downtime. Distributors receive access to a technical portal for update tracking and service ticket management. |
Need a Quote for Emerald Scanner Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


