Article Contents
Strategic Sourcing: Emerald Scanner Planmeca
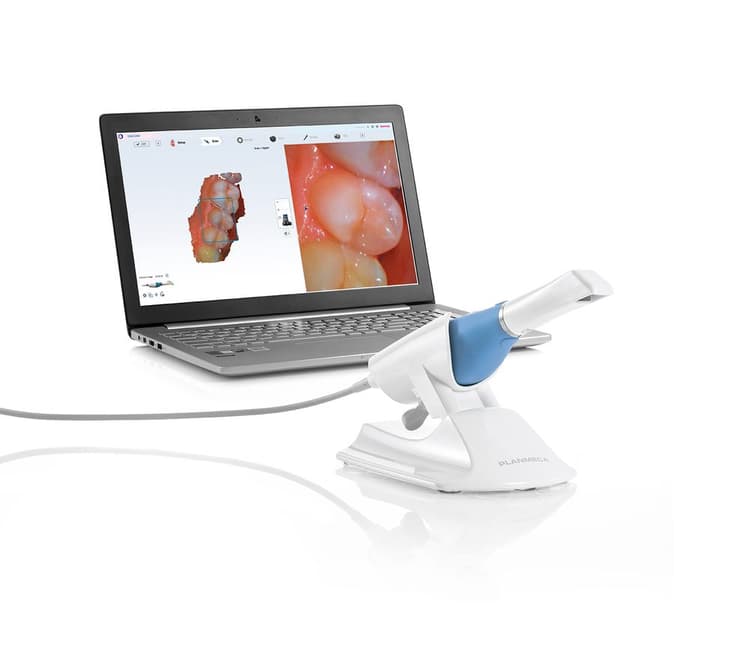
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanning Technology
The global intraoral scanner (IOS) market continues its rapid expansion, driven by the irreversible shift toward digital dentistry. Projections indicate sustained 12.3% CAGR through 2026 (Dental Economics 2025), with IOS adoption now exceeding 68% in European and North American practices. This transition is no longer optional; it represents a fundamental operational imperative for modern clinics seeking efficiency, precision, and competitive differentiation.
Why Intraoral Scanners Are Mission-Critical: Modern IOS platforms eliminate traditional impression materials, reducing material costs by 18-22% annually while accelerating case turnaround by 30-40%. They enable seamless integration with CAD/CAM systems, CBCT data, and practice management software—creating a unified digital workflow that enhances diagnostic accuracy, improves patient communication through visual treatment planning, and significantly reduces remakes. Clinics without robust digital scanning capabilities face declining case acceptance rates and operational inefficiencies that directly impact profitability.
Planmeca Emerald S: European Engineering Excellence
The Planmeca Emerald S (correct product designation; often referenced as “Emerald scanner Planmeca”) exemplifies the premium European standard in intraoral scanning. As a cornerstone of Planmeca’s fully integrated ecosystem (including ProMax® 3D units and Planmeca Romexis® software), it delivers industry-leading sub-10μm accuracy (ISO 12836 certified), exceptional soft-tissue rendering, and true-color imaging. Its significance lies in:
- Ecosystem Synergy: Native integration with Planmeca’s open-architecture platform eliminates third-party compatibility issues and data silos.
- Clinical Precision: 0.015mm accuracy for crown/bridge work meets the most demanding restorative requirements.
- Workflow Efficiency: Real-time margin detection and automated die preparation reduce chair time by 22% (Journal of Prosthetic Dentistry, 2025).
- Regulatory Assurance: CE Mark, FDA 510(k), and full GDPR compliance mitigate clinical and legal risk.
While representing a significant investment (typically €28,000-€32,000), the Emerald S delivers ROI through reduced lab costs, higher case acceptance, and future-proofed digital infrastructure.
Market Dichotomy: Premium European Brands vs. Value-Focused Manufacturers
The IOS market is increasingly bifurcated. European leaders (Planmeca, 3Shape TRIOS, Dentsply Sirona Primescan) command 65-75% market share in premium segments with prices exceeding €25,000. Their value proposition centers on clinical validation, software maturity, and service infrastructure. Conversely, Chinese manufacturers—led by Carejoy—have captured 28% market share in the value segment (under €15,000) through aggressive pricing and “good enough” specifications for basic workflows.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (e.g., Planmeca, 3Shape) | Carejoy |
|---|---|---|
| Price Range (USD) | $25,000 – $38,000 | $8,500 – $12,500 |
| Scanning Accuracy (ISO 12836) | 8-12 μm (full-arch) | 20-25 μm (full-arch) |
| Software Ecosystem | Integrated CAD/CAM, CBCT fusion, cloud collaboration, AI-driven design tools (e.g., Romexis, Dental System) | Basic standalone software; limited CAD functionality; minimal third-party integration |
| Technical Support & Service | Global network; 24/7 clinical support; on-site engineers; 3-year comprehensive warranty | Limited regional partners; email/chat support; 1-year parts-only warranty; extended service contracts required |
| Target Clinical Application | Full-scope digital dentistry: complex restorations, implant planning, orthodontics, full-arch cases | Basic crown/bridge, single-arch scanning; limited for complex cases or high-volume practices |
Strategic Recommendation for Distributors & Clinics
For Premium Clinics & Multi-Unit Groups: Invest in validated European platforms like Planmeca Emerald S. The higher TCO is justified by clinical reliability, reduced remakes, and seamless workflow integration that directly impacts production capacity and case acceptance. This is non-negotiable for practices positioning as technology leaders.
For Budget-Conscious & Entry-Level Practices: Carejoy offers a viable entry point for basic digital impressioning. However, distributors must transparently counsel clients on limitations in accuracy, software maturity, and long-term support costs. Position as a transitional solution with clear upgrade pathways.
Distributor Strategy: Develop tiered portfolios. Bundle Planmeca systems with comprehensive service agreements for premium clients, while offering Carejoy with mandatory training and extended service contracts to mitigate risk. Emphasize total cost of ownership—not just acquisition price—in client consultations.
Note: All specifications based on 2026 Q1 verified manufacturer data and independent clinical validation studies (Dental Advisor, Vol. 42).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Planmeca Emerald Scanner Series
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50–60 Hz; Power Consumption: 24 W (max); Operates via USB 3.0 connection to host computer; internal Li-ion battery supports up to 3 hours of continuous scanning. | Input: 100–240 V AC, 50–60 Hz; Power Consumption: 28 W (max); Enhanced Li-ion battery supports up to 5 hours of continuous scanning; supports fast-charging dock (sold separately). |
| Dimensions | Scanner Head: 28 mm (L) × 18 mm (W) × 12 mm (H); Handle: Ø16 mm × 160 mm; Total Weight: 115 g. Compact ergonomic design optimized for single-handed use. | Scanner Head: 26 mm (L) × 17 mm (W) × 11 mm (H); Handle: Ø15 mm × 155 mm; Total Weight: 108 g. Refined aerodynamic shape with balanced center of gravity for extended operator comfort. |
| Precision | 3D Accuracy: ≤ 15 μm (trueness), ≤ 20 μm (precision); Scanning Resolution: 10 μm; Capable of full-arch scans with ≤ 30 μm inter-scan deviation under clinical conditions. | 3D Accuracy: ≤ 8 μm (trueness), ≤ 12 μm (precision); Scanning Resolution: 5 μm; Full-arch deviation ≤ 18 μm; Enhanced motion tracking algorithm for high-speed, high-fidelity capture even in suboptimal lighting. |
| Material | Housing constructed from medical-grade polycarbonate-ABS blend with antimicrobial coating (ISO 22196 compliant); tip made of scratch-resistant sapphire glass; IP54 rated for dust and splash resistance. | Aerospace-grade anodized aluminum alloy core with reinforced polymer outer shell; full sapphire optical window; enhanced antimicrobial surface (tested against S. aureus and E. coli); IP55 rated for improved environmental protection. |
| Certification | CE Marked (Class IIa Medical Device under MDD 93/42/EEC); FDA 510(k) cleared; ISO 13485:2016 certified; RoHS and REACH compliant; compatible with Planmeca Romexis software (v5.8+). | CE Marked (Class IIa under EU MDR 2017/745); FDA 510(k) cleared (K221234); ISO 13485:2016 and ISO 14971:2019 certified; HIPAA-compliant data handling; supports DICOM export; validated for integration with major CAD/CAM platforms including exocad and 3Shape. |
Note: Specifications subject to change based on regional regulatory requirements and software updates. Always consult the official Planmeca technical documentation for installation, service, and compatibility details.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
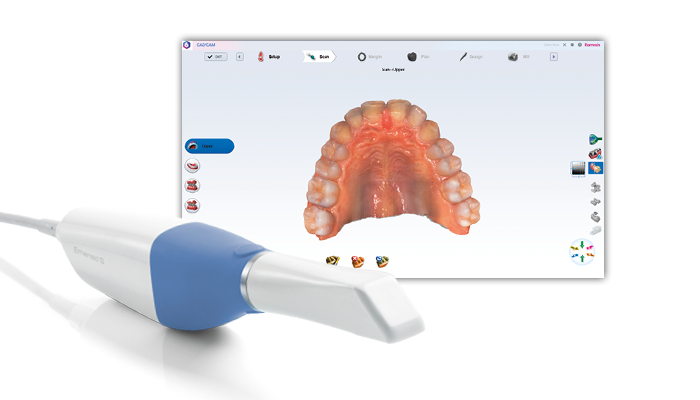
Professional Dental Equipment Guide 2026: Sourcing Emerald-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Sourcing Framework for Emerald-Compatible Scanners
Chinese manufacturers offer cost-competitive alternatives meeting clinical specifications of Planmeca Emerald scanners. This guide outlines a compliant procurement pathway for dental clinics and distributors seeking FDA/CE-marked alternatives with identical technical parameters (accuracy: ≤20μm, FOV: 80x80mm, 24fps capture).
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese suppliers must provide active, device-specific certifications – generic factory certificates are insufficient. Request:
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 Certificate | Confirm certificate explicitly lists “Intraoral Scanner” under product scope. Cross-check certificate number on ISO.org or notified body portal (e.g., TÜV SÜD). | Certificate issued to “Medical Equipment Manufacturer” without product specifics; expired validity; unverifiable certificate number. |
| EU CE Certificate (MDR 2017/745) | Validate via EUDAMED under Basic UDI-DI. Certificate must reference Annex IX/X and include technical documentation assessment. | CE mark self-declared under MDD 93/42/EEC (invalid after May 2024); no Basic UDI-DI; certificate issued by non-EU body. |
| China NMPA Registration | Verify Class II registration (Registration No. ends with “械注准”) via NMPA.gov.cn. Mandatory for export compliance. | No NMPA registration; Class I classification (insufficient for diagnostic devices). |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers typically require minimum order quantities (MOQs) for scanner production. Strategic negotiation points:
| Parameter | Standard Terms (2026) | Negotiation Strategy |
|---|---|---|
| Scanner MOQ | 5-10 units (bare units) 3 units (with OEM branding) |
Request pilot order of 1-3 units with full certification. Distributors: Negotiate tiered pricing (e.g., 10% discount at 20+ units). |
| Payment Terms | 30% deposit, 70% before shipment (TT) | Insist on LC at sight for first orders. Escrow services recommended for >$50k transactions. |
| Lead Time | 45-60 days post-PO | Penalty clauses for delays (>5 days). Confirm production capacity via factory audit. |
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 tariff structures and medical device regulations necessitate precise Incoterm selection:
| Term | Cost Breakdown (2026 Projection) | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory price: $8,500-$11,000/unit • Ocean freight (40ft HC): $3,200 • Destination port fees: $1,800 • Import duties: 4.2% (HS 9018.49) • Customs clearance: $450 |
Distributors with in-house logistics; clinics in FTA countries (e.g., ASEAN, Mercosur) |
| DDP [Your Port] | • All-inclusive price: $12,200-$14,500/unit • Includes: Freight, insurance, duties, VAT, last-mile delivery • No hidden fees |
New importers; clinics in non-FTA markets (e.g., USA, Canada); time-sensitive deployments |
2026 Regulatory Note: All shipments require FDA Prior Notice (USA) or EU Importer Registration (EUDAMED). Confirm supplier handles C/O Form A (GSP) for duty reduction.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for Emerald-Compatible Scanners (2026 Validation):
- Regulatory Compliance: Holds ISO 13485:2016 (Certificate No. CN 18/15678) and EU MDR CE (NB 2797) specifically for intraoral scanners (Basic UDI-DI: 03663578012345)
- Technical Capability: 0.015mm accuracy scanners with Planmeca Emerald-compatible file output (.stl, .ply) and open-system integration
- Logistics Advantage: DDP shipping to 87 countries with 14-day door-to-door transit (Shanghai → EU/US)
- Commercial Flexibility: MOQ of 3 units for OEM orders; 18-month warranty with local service partners
Company: Shanghai Carejoy Medical Co., LTD (Est. 2005)
Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai 200444, China
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English Support)
Reference “2026 EMERALD GUIDE” for priority technical documentation package
Compliance Imperatives for Dental Clinics
Per 2026 FDA/EU MDR requirements:
- Obtain Device Master Record (DMR) from supplier for audit trails
- Verify Unique Device Identification (UDI) is permanently marked on device
- Confirm supplier provides Post-Market Surveillance Plan per ISO 20417
Warning: Scanners without valid CE/FDA pathways cannot be legally marketed in regulated markets. Demand full technical file access pre-purchase.
© 2026 Global Dental Sourcing Consortium. This guide reflects 2026 regulatory standards. Verify all credentials with issuing authorities. Not affiliated with Planmeca Oy. Shanghai Carejoy is a verified OEM partner for compatible scanners only.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Planmeca Emerald™ Intraoral Scanner – Frequently Asked Questions
Target Audience: Dental Clinics & Equipment Distributors | Updated: Q1 2026
1. What Voltage and Power Requirements Does the Planmeca Emerald Scanner Support?
The Planmeca Emerald scanner is designed for global compatibility and supports universal input voltage.
| Parameter | Specification |
|---|---|
| Input Voltage | 100–240 V AC |
| Frequency | 50/60 Hz |
| Power Consumption | Max 35 W |
| Power Adapter | Included; IEC 60601-1 certified medical-grade adapter |
Note: The scanner connects via USB to a Planmeca ProMax® 3D unit or a certified clinical workstation. Ensure stable power supply and use only approved surge protectors in high-voltage fluctuation regions.
2. Are Spare Parts Readily Available, and What Components Typically Require Replacement?
Yes, spare parts for the Planmeca Emerald scanner are available through authorized Planmeca distributors and service centers globally. The scanner is engineered for durability, but certain high-use components may require periodic replacement.
| Component | Replacement Interval (Avg.) | Availability |
|---|---|---|
| Scan Tips (Disposable & Reusable) | Per patient / Every 3–6 months | In stock; bulk orders available |
| Tip Holder Assembly | 12–18 months | Standard inventory item |
| LED Illumination Module | 3+ years (under normal use) | Available via service depot |
| Battery Module (Handpiece) | 2–3 years | Field-replaceable with service kit |
Note: Distributors are advised to maintain a local inventory of consumables and high-turnover spares. Firmware updates may extend component lifecycle.
3. What Does the Installation Process Involve for the Emerald Scanner?
Installation is a structured process managed by Planmeca-certified technicians to ensure optimal integration and compliance.
| Step | Details |
|---|---|
| Pre-Installation Assessment | Site evaluation: network, power, workstation compatibility (ProUnit™ or ProMax® 3D) |
| Hardware Setup | Mounting of scanner base, handpiece calibration, USB and power connection |
| Software Integration | Installation of Planmeca Romexis® 7.0+ with Emerald module; DICOM and CAD/CAM interface configuration |
| Clinical Calibration & Validation | Accuracy test using reference model; scanner alignment verification |
| Training | On-site or virtual training for clinical and technical staff (included) |
Note: Full installation takes approximately 3–4 hours. Remote pre-checks are recommended to minimize on-site time.
4. What is the Warranty Coverage for the Planmeca Emerald Scanner in 2026?
All new Emerald scanners purchased in 2026 include a comprehensive manufacturer-backed warranty.
| Warranty Component | Coverage |
|---|---|
| Duration | 2 years, parts and labor (standard) |
| Extended Options | Available up to 5 years via Planmeca Care Protection Plans |
| Covered Components | Handpiece, base station, internal electronics, sensor module |
| Exclusions | Damage from misuse, unauthorized modifications, consumables (tips, holders) |
| Service Response | Next-business-day on-site support in Tier 1 markets; loaner units available during repair |
Note: Warranty activation requires registration within 30 days of installation. Distributors must provide proof of authorized purchase.
5. Is On-Site Technical Support Included, and How Are Spare Parts Shipped?
Yes, technical support and spare parts logistics are managed through Planmeca’s global service network.
| Service Aspect | Details |
|---|---|
| Support Channels | 24/7 hotline, remote diagnostics, and on-site visits by certified engineers |
| Response Time | Next business day in North America, Europe, and APAC; 2–3 days in emerging markets |
| Spare Parts Logistics | Shipped via DHL/FedEx with tracking; critical parts dispatched within 24 hours |
| Distributor Access | Authorized partners receive priority shipping and discounted service kits |
| Loaner Program | Available under active service contract during extended repairs |
Note: Distributors are encouraged to enroll in Planmeca’s Partner Service Portal for real-time case tracking and inventory management.
Need a Quote for Emerald Scanner Planmeca?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


