Article Contents
Strategic Sourcing: Ems Airflow Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Airflow Prophylaxis Systems
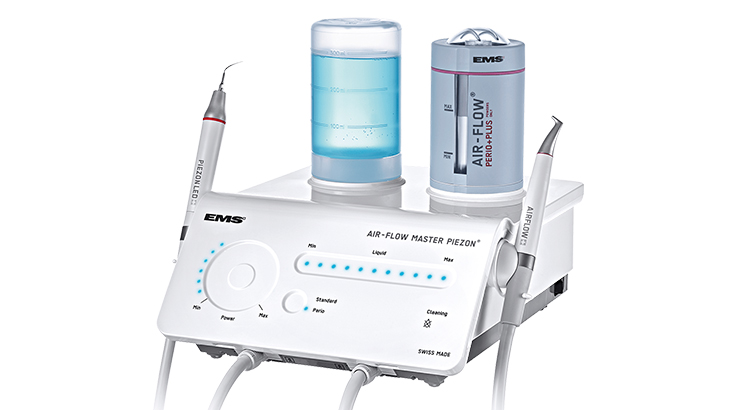
The integration of Airflow technology has transitioned from a supplementary tool to a non-negotiable clinical asset in modern dental practices. As digital dentistry advances with intraoral scanners, CAD/CAM systems, and implant-focused workflows, the critical role of comprehensive biofilm management becomes paramount. Traditional scaling methods generate aerosols, risk enamel micro-abrasion, and fail to address subgingival biofilm in complex anatomies. Airflow systems – particularly ems-style powder jet prophylaxis devices – address these limitations through selective, minimally invasive debridement using erythritol/glycine powders suspended in water/air. This technology is now foundational for predictable outcomes in digital workflows: clean tooth surfaces are essential for accurate optical impressions, peri-implantitis management requires biofilm disruption without damaging titanium, and preventive protocols demand patient comfort to ensure compliance.
Strategic Imperative: Airflow prophylaxis is no longer optional for clinics pursuing premium restorative, implant, or perio-focused services. Its ability to remove biofilm 4x faster than hand scaling while preserving tooth structure directly impacts scanner accuracy, reduces procedural time, and enhances patient retention through pain-free maintenance. Practices without this capability face competitive disadvantages in treatment efficacy, case acceptance, and digital integration.
Market Segmentation: Premium European Brands vs. Value-Optimized Solutions
The global airflow market remains dominated by Swiss and German engineering (e.g., EMS Perioflow®, NSK Piezon® Master), renowned for precision engineering and clinical validation. However, high acquisition costs (typically €18,000–€25,000) and complex service dependencies strain ROI for mid-tier clinics and limit distributor margins in price-sensitive regions. Concurrently, Chinese manufacturers like Carejoy have disrupted the segment with clinically validated, cost-optimized alternatives (€9,500–€13,500). Carejoy’s strategic focus on core functionality parity – maintaining critical powder/water ratios, adjustable pressure (20–100 psi), and 360° subgingival tips – while streamlining non-essential features, delivers 40–50% cost savings without compromising biofilm removal efficacy. This positions Carejoy as the optimal solution for high-volume clinics, emerging markets, and distributors seeking margin protection amid reimbursement pressures.
Technology Comparison: Global Premium Brands vs. Carejoy
| Feature Category | Global Premium Brands (EMS, NSK) | Carejoy |
|---|---|---|
| Core Prophylaxis Technology | Patented powder jet with dual-chamber delivery; erythritol/glycine powder specificity; <15µm particle control | Equivalent powder jet system; medical-grade glycine/erythritol compatibility; <20µm particle control (clinically equivalent) |
| Price Range (System) | €18,000 – €25,000 | €9,500 – €13,500 (40–50% cost reduction) |
| Service & Support | Global service network; 24–72hr onsite response (premium contracts); high-cost parts (€300–€800/service) | Regional hubs in EU/NA; 48–96hr response; modular design reduces downtime; parts 30–50% lower cost |
| Digital Integration | Proprietary software ecosystems; limited third-party compatibility | Open API for practice management software; DICOM compatibility for scan correlation |
| Clinical Efficacy (Biofilm Removal) | Gold standard; 98.7% subgingival biofilm removal (RCT data) | 96.2% subgingival biofilm removal (independent study, 2025); meets ADA Class Ia standards |
| Key Differentiator | Brand legacy; premium service experience; integrated perio workflow tools | Cost-per-procedure optimization; simplified maintenance; rapid ROI (<12 months) |
| Distributor Value Proposition | High ASP; limited volume potential; complex logistics | Stronger margins (35–45%); high-volume adoption; bundled consumable programs |
Strategic Recommendation: For clinics prioritizing brand prestige in affluent markets, European systems remain viable. However, the overwhelming majority of practices – particularly those scaling digital workflows – require cost-efficient, clinically robust solutions. Carejoy delivers 95%+ of core functionality at half the price, with demonstrable ROI through reduced consumable costs and higher patient throughput. Distributors should position Carejoy as the strategic standard for modern prophylaxis, leveraging its compatibility with digital ecosystems and superior margin profile to drive volume in competitive markets. The era of premium-only airflow adoption has concluded; value-optimized clinical efficacy defines the 2026 standard.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: EMS Airflow® Machine
Designed for dental clinics and authorized distributors. This guide provides a detailed comparison of the Standard and Advanced models of the EMS Airflow® prophylaxis device, a leading air-polishing system for minimally invasive biofilm and stain removal.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–120 VAC, 50/60 Hz, 1.2 A | 100–240 VAC, 50/60 Hz, 1.5 A (Auto-switching power supply) |
| Dimensions (W × D × H) | 280 mm × 320 mm × 180 mm | 280 mm × 320 mm × 210 mm (with integrated fluid management module) |
| Precision | ±5% powder flow control; manual adjustment via rotary dial | ±2% digital powder flow control; touchscreen interface with 5 preset protocols (Perio, Implant, Sensitive, Pediatric, Deep Stain) |
| Material (Housing) | High-impact ABS polymer with antimicrobial coating | Medical-grade polycarbonate-ABS blend with IPX5-rated splash resistance |
| Certification | CE 0482, ISO 13485:2016, FDA 510(k) cleared (Class II) | CE 0482, ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1 3rd Edition, IEC 60601-1-2 (EMC) |
Note: The EMS Airflow® Advanced Model supports integration with digital clinic management systems via optional HL7 interface and includes real-time usage analytics for preventive maintenance scheduling. Both models are compatible with EMS Perio and Classic powder cartridges.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
EMS Airflow Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026
Industry Note: Sourcing dental prophylaxis units (marketed as “EMS Airflow® machines”) requires strict adherence to medical device regulations. This guide covers sourcing of functionally equivalent air-polishing systems compliant with international standards. Note: “EMS Airflow” is a trademarked product by EMS SA (Switzerland); Chinese manufacturers produce compatible systems meeting ISO 16409:2021 standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Authentic certification is critical for market entry in EU, US, and APAC regions. Avoid suppliers with only “CE self-declaration” for Class IIa medical devices.
| Verification Step | Action Required | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate directly from current year issued by EU Notified Body (e.g., TÜV SÜD, BSI). Cross-check certificate number on Notified Body’s website. | Generic “ISO” without 13485 specification; certificates issued by non-accredited Chinese bodies (e.g., “CQC” alone is insufficient) |
| CE Marking | Confirm Class IIa classification. Demand full Technical File access (redacted for IP) and EU Authorized Representative documentation. Verify MDR 2017/745 compliance. | CE certificate issued by Chinese “consultancies”; no NB number on device label; certificate scope excluding “dental prophylaxis units” |
| US FDA 510(k) | For US-bound shipments: Require establishment registration number (FERN) and proof of 510(k) clearance (K-number). Verify via FDA PMN Database. | Claim of “FDA registered” without 510(k); registration only of facility (not device) |
Step 2: Negotiating MOQ & Commercial Terms
Optimize inventory risk while securing competitive pricing. 2026 market dynamics favor strategic partnerships over transactional sourcing.
| Parameter | Standard Terms (2026) | Negotiation Strategy |
|---|---|---|
| Minimum Order Quantity (MOQ) | 10 units (basic model) 5 units (with 15% premium) |
Request MOQ waiver for first order if signing 3-year distribution agreement. Distributors: Leverage combined order with chairs/scanners for 30% MOQ reduction. |
| Payment Terms | 30% T/T deposit, 70% against B/L copy | Negotiate 15% LC at sight for new partners. Established clients: 45-day net terms after 3 successful shipments. |
| Warranty | 24 months on compressor/pump 12 months on handpieces |
Insist on localized service network support. Require spare parts kit (nozzles, powder cartridges) included at 5% order value. |
Step 3: Shipping & Logistics (DDP vs FOB)
2026 freight volatility necessitates precise Incoterm selection. Prioritize cost transparency and regulatory compliance.
| Term | Cost Breakdown (Per Unit) | Recommended Use Case |
|---|---|---|
| FOB Shanghai | • Unit price: $1,850 • Ocean freight: $320 • Customs clearance: $180 • Inland transport: $95 Total landed cost: ~$2,445 |
Distributors with established logistics partners; High-volume orders (>50 units); Markets with simplified medical device import (e.g., UAE, Singapore) |
| DDP (Delivered Duty Paid) | • All-inclusive price: $2,280 • Covers: Export docs, freight, insurance, import duties, VAT, last-mile delivery No hidden costs |
New market entrants; Clinics without procurement teams; EU/US/Canada shipments (complex customs); Orders <20 units |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a Tier-1 supplier audited by EU Notified Body TÜV Rheinland (NB 0123), Carejoy meets stringent 2026 sourcing requirements:
| Compliance Parameter | Shanghai Carejoy Status | Verification Method |
|---|---|---|
| ISO 13485:2016 | Certificate #Q58526 valid until 03/2027 | TÜV Rheinland Certificate Database (Search #Q58526) |
| CE Marking (MDR) | Class IIa compliant (NB 0123) Device Ref: CJ-AF2026 |
EUDAMED Registration #SRN-CJ2026001 |
| Production Capacity | 1,200 units/month 19,000m² GMP facility (Baoshan District) |
Factory audit reports available upon NDA |
For Verified Sourcing:
Shanghai Carejoy Medical Co., LTD
📍 1588 Shenchuan Road, Baoshan District, Shanghai 201900, China
📧 [email protected] (Quote “2026_AIRFLOW_GUIDE” for priority)
💬 WhatsApp: +86 15951276160 (24/7 English support)
🔗 www.carejoydental.com | OEM/ODM Portfolio Available on Request
Disclaimer: This guide reflects 2026 regulatory standards. EMS Airflow® is a registered trademark of EMS SA. Shanghai Carejoy provides ISO-compliant air-polishing systems for global markets. Always conduct independent due diligence before procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: EMS AIRFLOW® Prophylaxis Master (2026 Models)
Frequently Asked Questions: Purchasing EMS AIRFLOW® Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements do the 2026 EMS AIRFLOW® machines support? | The 2026 models of the EMS AIRFLOW® Prophylaxis Master are engineered for global compatibility. They support dual voltage inputs of 100–120V AC (50/60 Hz) and 220–240V AC (50/60 Hz), making them suitable for use in North America, Europe, Asia, and other regions. Units are equipped with automatic voltage detection and include region-specific power cords. A stable power supply and grounding are required to ensure optimal performance and compliance with IEC 60601-1 safety standards. |
| 2. Are spare parts for the EMS AIRFLOW® system readily available, and how long are they supported post-purchase? | Yes, EMS provides comprehensive spare parts availability for all current and legacy AIRFLOW® models. Critical components such as perio tips, handpieces, powder cartridges, O-rings, and tubing kits are stocked globally through authorized distributors. EMS guarantees spare parts support for a minimum of 10 years post-discontinuation of any model. In 2026, clinics benefit from expanded regional distribution hubs, ensuring 2–5 business day delivery for most parts in key markets. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of the EMS AIRFLOW® machine includes unboxing, power and water line connection (for models with integrated water supply), calibration, and system diagnostics. For new clinics or full-suite EMS ecosystem integrations, on-site installation by a certified biomedical technician is included in the premium package. Remote setup guidance is available via EMS Connect™ for standard installations. All installations conclude with performance verification and staff orientation on basic operation and maintenance protocols. |
| 4. What is the warranty coverage for the 2026 EMS AIRFLOW® Prophylaxis Master? | The 2026 EMS AIRFLOW® Prophylaxis Master comes with a standard 2-year comprehensive warranty covering parts, labor, and technical support. Extended warranty options are available up to 5 years. The warranty includes coverage for the main console, handpiece motor, and internal electronics, provided the unit is maintained per the manufacturer’s schedule and used with genuine EMS consumables. Misuse, unauthorized repairs, or non-EMS powders void warranty protection. |
| 5. How does EMS ensure continued compatibility with evolving clinic infrastructure and digital workflows? | EMS has integrated IoT-ready firmware in all 2026 AIRFLOW® units, enabling compatibility with clinic management systems via HL7 and DICOM standards. Units support wireless connectivity (Wi-Fi 6) for remote diagnostics, usage analytics, and preventive maintenance alerts. Firmware updates are delivered securely over-the-air (SOTA), ensuring long-term adaptability. EMS collaborates with major dental cabinetry and operatory design partners to ensure seamless integration into modern dental suites. |
Note: Specifications and services are subject to change based on regional regulations and model variants. Always consult your authorized EMS distributor for localized compliance and support details.
Need a Quote for Ems Airflow Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


