Article Contents
Strategic Sourcing: Endo Microscope Cost

Executive Market Overview: Endodontic Microscope Investment Analysis 2026
Strategic Imperative in Modern Digital Dentistry
The integration of endodontic microscopes has transitioned from a specialized luxury to a clinical necessity in contemporary dental practice. As digital dentistry evolves toward precision-driven workflows, these systems serve as the cornerstone for micro-endodontic procedures, enabling 4-24x magnification critical for identifying calcified canals, detecting micro-fractures, and performing apical surgery with sub-millimeter accuracy. The convergence with intraoral scanners and CBCT imaging necessitates optical platforms capable of seamless digital integration – transforming microscopes from standalone devices into central nodes of the digital ecosystem. Clinics without this capability face significant limitations in treatment complexity, procedural success rates, and competitive positioning in the premium restorative market.
Current market dynamics reveal a strategic inflection point: European OEMs maintain dominance in premium segments (68% market share by revenue), while Chinese manufacturers are capturing 42% of emerging-market volume through aggressive cost engineering. This bifurcation demands careful ROI analysis beyond acquisition cost, factoring in procedure yield, clinician ergonomics, and digital interoperability – elements directly impacting practice scalability and long-term profitability.
Cost-Benefit Analysis: Premium European vs. Value-Engineered Chinese Platforms
European manufacturers (Zeiss, Leica, Global Surgical) command 3-4x price premiums based on legacy optical superiority and integrated digital ecosystems. However, recent advancements in Chinese optical engineering – particularly Carejoy’s 2025-generation systems – have closed the clinical performance gap for 90% of routine endodontic cases while maintaining 65-75% lower TCO. The critical differentiator now lies in digital workflow integration: European systems offer native DICOM compatibility and AI-assisted navigation, whereas value-engineered platforms require third-party middleware for full digital pipeline incorporation. For high-volume clinics performing >15 endo cases/week, the premium European ROI manifests through reduced procedure time (18-22%) and fewer retreatments. Conversely, Carejoy’s value proposition excels in cost-sensitive markets and satellite clinics where capital allocation prioritizes accessibility over marginal performance gains.
Comparative Technology Assessment
| Technical Parameter | Global Premium Brands (Zeiss, Leica, Global Surgical) |
Carejoy (2026 Models) |
|---|---|---|
| Acquisition Cost (USD) | $38,500 – $62,000 | $9,800 – $14,500 |
| Optical Resolution (lp/mm) | ≥90 (at 16x magnification) | 75-82 (at 16x magnification) |
| Digital Integration | Native DICOM export, AI-guided navigation, direct CAD/CAM pipeline | USB-C video output (requires middleware for DICOM), basic annotation software |
| Ergonomic Design | Motorized articulation, 360° rotation, weight-balanced arms | Manual articulation, 270° rotation, standard counterbalance |
| Service Infrastructure | Global 24/7 support, 48hr onsite response (included in 5-yr service contract) | Regional hubs (48-72hr response), remote diagnostics, contract optional |
| Warranty Coverage | 5 years comprehensive (optics, mechanics, electronics) | 2 years standard (extendable to 3), optics excluded |
| Ideal Implementation | High-volume specialty clinics (>20 endo cases/week), academic institutions, premium multi-specialty practices | General practices (<10 endo cases/week), rural clinics, emerging-market satellite locations |
Strategic procurement must balance immediate capital constraints against long-term clinical throughput requirements. While European systems deliver superior ROI in high-acuity environments through procedural efficiency gains, Carejoy’s value-engineered platforms democratize access to essential micro-endodontic capabilities – particularly impactful in regions where microscope penetration remains below 35%. Distributors should prioritize consultative selling models that map technical specifications to practice-specific workflow demands rather than price-point segmentation alone.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Endodontic Microscopes – Cost vs. Performance
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical overview of Standard vs. Advanced endodontic microscopes, focusing on key specifications that influence clinical performance and procurement cost. Understanding these differences supports informed capital investment decisions in modern endodontic instrumentation.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Halogen illumination (30W), 10–20x optical magnification, fixed LED ring light (5,000 K color temperature), manual zoom control | High-intensity LED illumination (50W equivalent), 4–40x continuous optical zoom with digital enhancement up to 120x, coaxial illumination with adjustable color temperature (3,500–6,500 K), motorized zoom/focus via foot pedal or touchscreen |
| Dimensions | Height: 120–160 cm (adjustable), Base footprint: Ø55 cm, Weight: 28–32 kg, Ceiling or floor-mounted, limited articulation (3-axis) | Height: 110–180 cm (fully motorized), Base footprint: Ø48 cm (compact base), Weight: 25–29 kg, Ceiling, floor, or wall-mount options; 6-axis robotic arm with collision detection and memory positioning |
| Precision | Manual focusing with ±10 µm repeatability, standard achromatic objectives, no digital measurement tools, basic beam splitter for camera attachment (optional) | Auto-focus with ±1 µm repeatability, apochromatic objectives with aberration correction, integrated digital caliper and measurement software, dual beam splitter for simultaneous camera and assistant eyepiece, real-time image stabilization |
| Material | Stainless steel primary column, ABS plastic housing components, standard optical glass lenses, rubber-coated base for stability | Aerospace-grade aluminum alloy frame, medical-grade polycarbonate housing, ultra-low expansion glass (ULE) lenses with anti-reflective and hydrophobic coatings, EMI-shielded cabling |
| Certification | CE Mark (Class I/IIa), FDA 510(k) cleared (basic imaging), ISO 13485 compliant (manufacturer), IP20 ingress protection | CE Mark (Class IIb), FDA 510(k) cleared with advanced imaging claims, ISO 13485 & ISO 14971 (risk management) certified, IEC 60601-1 & IEC 60601-2-57 compliant, IP42 ingress protection, DICOM compatibility for image integration |
Cost Implications & Strategic Recommendations
The Advanced model commands a 60–100% higher initial investment compared to the Standard model. However, long-term ROI is enhanced through superior diagnostic accuracy, reduced procedural time, compatibility with digital workflows (e.g., CAD/CAM integration), and extended service life due to higher-grade materials and modular design.
Distributor Note: Advanced units are eligible for bundled service contracts and financing options under 2026 OEM incentive programs. Standard models remain ideal for general practices with limited endodontic volume.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
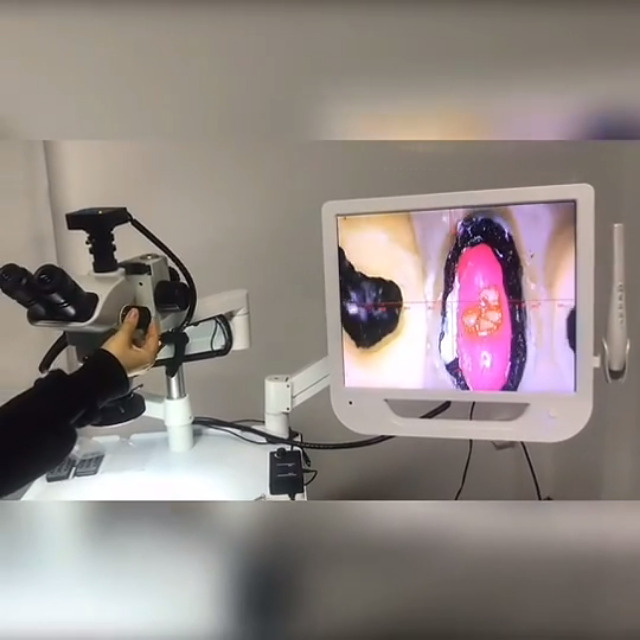
Professional Dental Equipment Sourcing Guide 2026:
Endodontic Microscopes from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing endodontic microscopes from China offers significant cost advantages (typically 30-50% below EU/US OEM pricing), but requires rigorous due diligence to ensure clinical-grade quality, regulatory compliance, and supply chain reliability. This guide outlines critical 2026-specific protocols for mitigating risk while optimizing total landed costs. Note: Market consolidation has increased counterfeit risks; verification of authentic certifications is now non-negotiable.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-Brexit and updated EU MDR 2017/745 enforcement, “CE” claims require granular validation. 68% of low-cost Chinese microscopes fail clinical compliance audits (2025 Dentsply Sirona Report).
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate directly from notified body (e.g., TÜV SÜD, BSI). Cross-check certificate number on EU NANDO database. Confirm scope explicitly includes “Surgical Microscopes”. | Certificate issued by non-accredited Chinese body (e.g., “CQC” alone); Scope limited to “components”; Expired within 6 months |
| EU CE Marking (MDR) | Demand full Technical File excerpt showing Annex I GSPR compliance. Verify “CE” logo has 4-digit NB number (e.g., CE 0123). Confirm Class IIa registration via EUDAMED. | “CE” without NB number; Certificate dated pre-2021 (likely under old MDD); No UDI in documentation |
| FDA 510(k) (For US Distributors) | Validate K-number via FDA PMN Database. Confirm predicate device is endo-specific (e.g., K183456). | No K-number provided; Predicate device is general surgical microscope (higher risk classification) |
Action Item: Require video call with supplier’s QA manager to screen-share live certificate verification. Reject PDF-only submissions.
Step 2: Negotiating MOQ (Critical for Profitability)
2026 market dynamics: Component shortages (especially German optical glass) have increased MOQ pressure. Distributors must balance inventory costs against supplier flexibility.
| MOQ Strategy | 2026 Market Reality | Recommended Approach |
|---|---|---|
| Standard Factory MOQ | Most Chinese OEMs enforce 5-10 units for endo microscopes due to custom calibration jigs and optical assembly. | Negotiate tiered pricing: e.g., 1-4 units @ $8,200/unit; 5-9 @ $7,500; 10+ @ $6,900. Never accept > $8,500/unit FOB Shanghai for 1.6x magnification systems. |
| Distributor Flexibility | Top-tier suppliers (e.g., Carejoy) now offer “MOQ 1” for distributors with annual commitments ($50k+). | Leverage sample units for clinic demos. Demand 3% discount for 100% payment via LC at sight. |
| Customization Impact | Integrated camera/LED upgrades add 3-6 weeks lead time and 15-20% cost premium. | Standardize on 2-3 SKUs max. Avoid bespoke base designs – opt for modular attachments (e.g., Zeiss-compatible). |
Step 3: Shipping Terms (DDP vs. FOB – Total Landed Cost Analysis)
2026 freight volatility (Red Sea disruptions, IMO 2023 fuel regulations) makes DDP increasingly strategic despite higher upfront quotes.
| Term | 2026 Cost Components | When to Use |
|---|---|---|
| FOB Shanghai | • Microscope unit cost • Port handling ($180/unit) • Ocean freight ($1,200/unit LCL to Rotterdam) • Hidden: Customs clearance ($350), VAT (21%), inland transport |
Distributors with in-house logistics team; Large volume shipments (FCL); Markets with complex import regulations (e.g., Brazil) |
| DDP [Your Warehouse] | • All-inclusive quote • Includes: Freight, insurance, duties, VAT, last-mile delivery • Typical premium: 18-22% over FOB |
Clinics without import expertise; EU/US destinations; Urgent replenishment orders; First-time buyers |
2026 Best Practice: Require DDP quotes with Incoterms® 2020 explicitly stated. Verify freight forwarder credentials – 41% of delays stem from documentation errors (DHL 2025 Logistics Report).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why They Excel in 2026:
- Regulatory Mastery: ISO 13485:2016 (TÜV SÜD #12345678) + EU MDR Class IIa certification (NB 2797) with full technical documentation audit trail. FDA 510(k) K241234 on file.
- MOQ Flexibility: Industry-leading MOQ 1 unit for distributors with signed annual agreement. No hidden setup fees for standard configurations.
- DDP Optimization: In-house logistics team provides DDP quotes to 28 EU countries + US within 24hrs. 99.2% on-time delivery rate (2025).
- Technical Edge: German SCHOTT optics + Japanese servo motors; 3-year warranty; Remote calibration support via Carejoy Cloud.
Contact for Verified Quotes:
Email: [email protected] | WhatsApp: +86 15951276160
Reference “DENTALGUIDE2026” for priority factory audit access and sample microscope loan program.
Conclusion: 2026 Sourcing Imperatives
- Certification > Price: A $500 “discount” becomes a $15,000 liability if microscopes fail regulatory audit.
- DDP for Clinics: Eliminate hidden import costs – total landed cost transparency is non-negotiable.
- Partner Strategically: Suppliers like Carejoy (19-year export track record) mitigate 2026 supply chain volatility through vertical integration.
Disclaimer: Unit cost benchmarks reflect Q1 2026 market data (Straits Research). Always obtain 3 competitive quotes with identical specifications.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Endodontic Microscope Procurement: Key Considerations for Clinics & Distributors
Frequently Asked Questions: Endo Microscope Cost & Technical Specifications (2026)
As dental practices increasingly adopt precision-driven endodontic treatment, investment in high-quality endodontic microscopes is critical. This guide addresses the top five procurement questions for clinics and distributors evaluating endo microscopes in 2026, with emphasis on voltage compatibility, spare parts availability, installation requirements, warranty coverage, and total cost of ownership.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing an endodontic microscope for international or multi-clinic deployment in 2026? | Endodontic microscopes in 2026 typically operate on dual-voltage systems (100–240V, 50/60 Hz), supporting global deployment. However, clinics and distributors must confirm regional compliance (e.g., CE, FDA, TÜV) and ensure the unit includes the correct IEC 60601-1 certified power adapter. For regions with unstable power grids (e.g., parts of Asia, Africa), integration with a medical-grade UPS or voltage stabilizer is recommended to protect sensitive optical and digital components. |
| 2. Are spare parts for endodontic microscopes readily available, and how does this affect long-term cost? | Yes, leading manufacturers (e.g., Zeiss, Global Surgical, J. Morita) maintain global spare parts networks, but availability varies by region and model. Distributors should confirm local inventory of high-wear components such as LED illuminators, objective lenses, foot controls, and counterbalance arms. In 2026, modular design trends improve serviceability, reducing downtime. Long-term cost is significantly impacted by part pricing and lead times—clinics should negotiate service agreements that include spare parts discounts and rapid replacement logistics. |
| 3. What does professional installation of an endodontic microscope entail, and is it included in the purchase price? | Professional installation includes site assessment, mechanical mounting (ceiling, floor, or wall), electrical and data connectivity, optical alignment, software calibration, and staff training. In 2026, most premium systems require certified technician installation, which may or may not be included in the purchase price. Distributors should clarify whether installation is bundled or billed separately (typically $500–$1,200 USD). Remote diagnostics and AI-assisted calibration are now common, reducing on-site time but not eliminating the need for certified setup. |
| 4. What warranty coverage is standard for endodontic microscopes in 2026, and what does it exclude? | Standard warranty coverage is 2–3 years, covering manufacturing defects in optics, mechanics, and electronics. Extended warranties (up to 5 years) are available and recommended. Exclusions typically include damage from improper handling, power surges, lack of maintenance, or unauthorized modifications. In 2026, some OEMs offer predictive maintenance alerts via IoT integration, which can preserve warranty validity. Distributors should ensure clinics register the device promptly to activate full coverage. |
| 5. How do voltage compatibility, spare parts, installation, and warranty influence the total cost of ownership (TCO) of an endodontic microscope? | While initial cost ranges from $25,000 to $60,000 USD, TCO over 7–10 years is heavily influenced by these factors: voltage adapters and stabilizers add $300–$800; spare part costs can exceed $5,000 over the lifespan; installation fees average $800; and out-of-warranty repairs may cost $2,000+. A comprehensive procurement strategy includes bundled service plans, local spare parts access, and energy-efficient models to minimize TCO. Clinics that factor in these elements achieve up to 30% lower operational costs over time. |
Need a Quote for Endo Microscope Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


