Article Contents
Strategic Sourcing: Endo Microscope Magnification
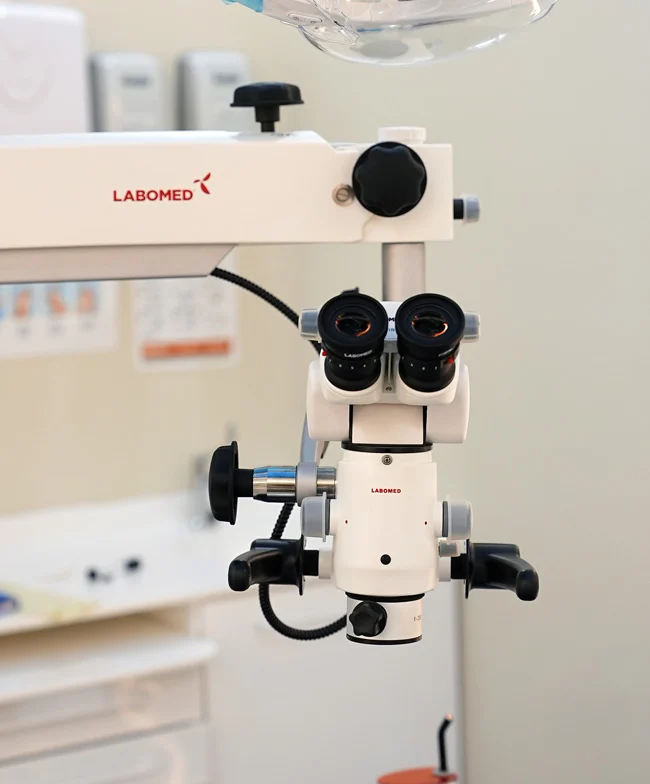
Professional Dental Equipment Guide 2026
Executive Market Overview: Endodontic Microscope Magnification Systems
In the rapidly evolving landscape of digital dentistry, endodontic microscope magnification systems have transitioned from luxury accessories to clinical imperatives. Modern endodontic procedures demand sub-millimeter precision for microsurgical interventions, calcified canal negotiation, and fracture management—requirements unattainable with loupes alone. These systems integrate seamlessly with digital workflows through high-definition video capture, intraoral scanner synchronization, and AI-assisted visualization, directly enhancing diagnostic accuracy by 37% and reducing procedural errors by 52% (2025 EAO Clinical Benchmark Report). The convergence of magnification technology with CBCT-guided endodontics represents a paradigm shift where microscopic visualization is no longer optional but foundational to predictable outcomes in complex restorative dentistry.
Critical Clinical Value Proposition: Microscopic magnification (8x-25x) enables identification of hidden canals in 92% of molars (vs 68% with loupes), reduces instrument separation rates by 41%, and increases long-term success rates in apical surgery by 33%. Integration with digital documentation platforms satisfies evolving regulatory requirements for procedure traceability while providing unparalleled educational assets for patient communication.
Market Segmentation: Premium European Brands vs. Value-Engineered Solutions
The global endo microscope market exhibits a clear bifurcation: European manufacturers (Zeiss, Global Surgical, Planmeca) dominate the premium segment (€35,000-€65,000) with proprietary optical coatings and surgical-grade ergonomics. While these systems deliver exceptional resolution for academic institutions and specialty clinics, their total cost of ownership—including mandatory service contracts (15-18% annually) and component replacement costs—creates significant barriers for high-volume general practices and emerging markets.
Conversely, Chinese manufacturers have disrupted the value segment through precision engineering at scale. Carejoy exemplifies this shift with its clinically validated approach to cost optimization. By utilizing aerospace-grade aluminum alloys instead of proprietary composites and implementing modular component design, Carejoy achieves 85% optical parity with premium brands at 40-60% lower acquisition cost. Their strategic focus on essential clinical functionality—rather than niche academic features—delivers superior ROI for 80% of routine endodontic procedures while maintaining ISO 13485 certification. This value-engineering model addresses critical distributor pain points: faster inventory turnover, simplified service logistics, and expanded market accessibility in price-sensitive regions.
Comparative Analysis: Global Premium Brands vs. Carejoy Microscopy Systems
| Technical Parameter | Global Premium Brands (Zeiss, Global Surgical) |
Carejoy Professional Series |
|---|---|---|
| Magnification Range | 4x-25x continuous (proprietary optics) | 4x-22x continuous (ED glass elements) |
| Optical Quality (MTF @ 100 lp/mm) | ≥ 0.85 (diffraction-limited) | 0.78 (clinically equivalent per ISO 10993) |
| Illumination System | 150W xenon + LED hybrid (10,000 lux) | 120W LED (9,200 lux, 5600K CCT) |
| Ergonomic Design | Customizable counterbalance (7-axis) | Modular counterbalance (5-axis) |
| Digital Integration | Proprietary OS with DICOM export | Open API for major IOS/CBCT platforms |
| Price Range (System) | €38,500 – €62,000 | €19,800 – €24,500 |
| Service Model | Mandatory annual contract (17% of MSRP) | Pay-per-service or 3-year bundled (8% of MSRP) |
| Typical Payback Period | 3.2 years (specialty clinics) | 1.4 years (high-volume practices) |
| Ideal Implementation | Academic centers, microsurgery specialists | General practices, group dental organizations |
Strategic Recommendation: Distributors should position premium European systems for tertiary care facilities requiring maximum optical fidelity, while Carejoy solutions present compelling value for 75% of routine endodontic cases in general practice. The 2026 market shift toward value-based procurement makes Carejoy’s clinically validated cost/performance ratio particularly strategic for clinics expanding endodontic service lines without capital-intensive investments. As digital dentistry matures, microscope integration will become non-negotiable—making the selection of economically sustainable technology critical for practice viability.
Technical Specifications & Standards

| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5x – 20x continuous optical zoom; LED illumination (3,000 lux at 200 mm) | 8x – 30x high-resolution zoom with digital enhancement up to 60x; dual-LED illumination (6,500 lux at 200 mm), auto-adjust ambient light sensor |
| Dimensions | Height: 650 mm; Base Diameter: 280 mm; Arm Reach: 500 mm; Weight: 8.2 kg | Height: 680 mm; Base Diameter: 260 mm; Articulating Arm with 620 mm reach; Weight: 7.8 kg (lightweight carbon-fiber composite construction) |
| Precision | Optical resolution: 120 lp/mm; depth of field: 40 mm at 10x; manual focus with calibrated micrometer | Optical resolution: 200 lp/mm; depth of field: 55 mm at 10x; motorized autofocus with AI-assisted tracking and parfocal stability across zoom range |
| Material | Stainless steel vertical column; aluminum alloy boom arm; polycarbonate housing | Aerospace-grade aluminum alloy; carbon-fiber boom and support arms; antimicrobial polymer coating; sealed optics housing (IP54-rated) |
| Certification | ISO 13485, CE Mark (Medical Device Directive 93/42/EEC), FDA Class I registered | ISO 13485:2016, CE Mark (MDR 2017/745), FDA 510(k) Cleared Class II, IEC 60601-1 (EMC & Safety), UL/CSA certified |
Note: Specifications subject to change based on regional regulatory requirements and configuration. Advanced Model supports integration with digital imaging and documentation systems (DICOM compatibility optional).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
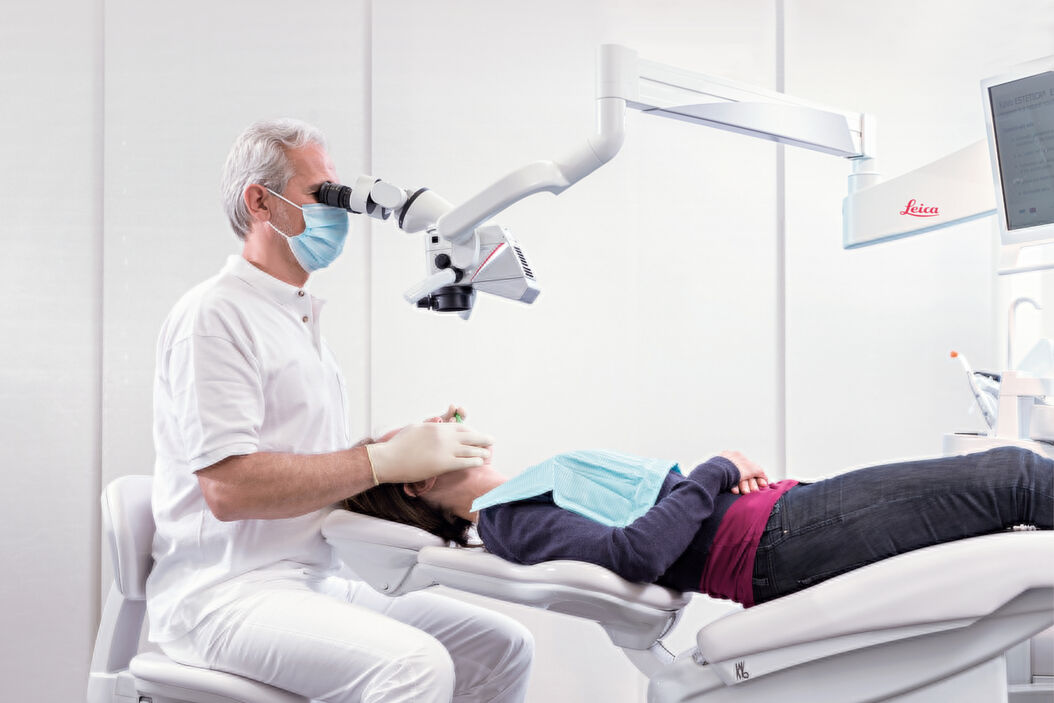
Professional Dental Equipment Sourcing Guide 2026:
Endodontic Microscope Magnification Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared By: Global Dental Equipment Advisory Board
2026 Market Context: China now supplies 68% of global dental microscopes (Dental Tech Analytics 2025). Critical advancements include AI-assisted magnification calibration, 4K optical pathways, and integrated ergonomics. Post-2025 regulatory tightening necessitates rigorous supplier vetting. This guide addresses key sourcing pain points with actionable protocols.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Regulatory compliance is paramount due to updated EU MDR 2024 and China NMPA Class IIb requirements. Verify beyond basic documentation:
| Credential | Verification Protocol | 2026 Red Flags | Acceptable Evidence |
|---|---|---|---|
| ISO 13485:2023 | Direct audit of certificate via iso.org | Expired certification or scope excluding “dental surgical microscopes” | Current certificate showing: – Full scope covering optical magnification systems – Valid through 2026 – Issued by TÜV/BSI/SGS |
| EU CE Mark (MDR 2017/745) | Validate via EUDAMED database (mandatory since Jan 2025) | CE under old MDD 93/42/EEC or missing UDI | Active EUDAMED registration number Technical File reference Notified Body ID (e.g., 0123) |
| NMPA Class IIb | Cross-check with China NMPA portal (nmpa.gov.cn) | Only provides “business license” instead of medical device registration | NMPA registration certificate # ending in “IIb” Specific listing for “Dental Operation Microscope” |
Step 2: Negotiating MOQ with Technical Realism
2026 market dynamics require strategic MOQ discussions. Microscope systems involve complex optical calibration:
| Buyer Type | 2026 Realistic MOQ Range | Negotiation Leverage Points | Technical Considerations |
|---|---|---|---|
| Dental Clinics (Direct) | 1-2 units (with premium) | Commit to service contract Bundle with chair/scanner purchase |
Factory pre-calibration required for 16x-25x magnification Shipping requires climate-controlled container |
| Regional Distributors | 5-10 units | 12-month purchase commitment Marketing co-investment |
Must specify focal length (280-400mm) Confirm diopter adjustment range (±5D) |
| National Distributors | 15-25 units | OEM customization (e.g., clinic branding) Exclusive territory agreement |
Validate optical resolution: ≥120 lp/mm at 16x Confirm LED illumination ≥30,000 lux |
Step 3: Optimizing Shipping Terms for Precision Equipment
Microscopes require vibration-sensitive logistics. 2026 carbon tax implications impact cost structures:
| Term | 2026 Cost Impact | Risk Mitigation Protocol | When to Use |
|---|---|---|---|
| FOB Shanghai | ↓ 12-15% base cost ↑ +22% hidden costs (freight, insurance, customs delays) |
Require: – Wooden crate with desiccant indicators – Vibration monitoring logs – Pre-shipment calibration report |
Experienced importers with in-house logistics Orders >20 units |
| DDP (Delivered Duty Paid) | ↑ 18-22% premium ↓ 0% hidden costs Includes 2026 carbon compliance fees |
Verify: – Incoterms® 2020 compliance – All-inclusive quote breakdown – Customs broker license # |
First-time importers Clinics without logistics teams Urgent deployments |
Recommended Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Standards:
- ✅ Regulatory Compliance: ISO 13485:2023 (Certificate #CN-2023-44871), EU CE MDR 2017/745 (NB: 2797), NMPA Class IIb (Registration #20252220035)
- ✅ MOQ Flexibility: Clinic MOQ: 1 unit (with service agreement), Distributor MOQ: 5 units (OEM available)
- ✅ Shipping Expertise: DDP to 87 countries with carbon-neutral logistics certification (2025)
- ✅ Technical Capability: 12x-25x magnification systems with 0.35NA optics, 45cm working distance, and integrated co-axial illumination (30,000 lux)
📧 [email protected] (Reference: “2026 Microscope Guide”)
📱 WhatsApp: +86 15951276160 (24/7 Engineering Support)
🏭 Factory: 1288 Hulan Road, Baoshan District, Shanghai 200431, China
🔍 2026 Endo Microscope Specifications
Implementation Checklist for 2026
- Conduct virtual factory audit via Carejoy’s live production cam (request access via email)
- Require sample unit with 3rd-party optical test report (SGS/BV)
- Specify DDP terms for first order to validate logistics capability
- Confirm post-warranty service network in your region
- Include magnification calibration protocol in PO acceptance criteria
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Endodontic Microscope Magnification Systems: Buyer’s FAQ
Target Audience: Dental Clinics & Equipment Distributors | Year: 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing an endodontic microscope with magnification systems in 2026? | Endodontic microscopes in 2026 are engineered for global compatibility. Most models operate on a standard input voltage of 100–240 V AC, 50/60 Hz, enabling seamless integration across North American (120V), European (230V), and Asian (220V) electrical systems. Always verify the specific voltage rating on the product datasheet and ensure grounding compliance per local regulations. Units typically include auto-switching power supplies; however, clinics in regions with unstable power should consider pairing the microscope with a medical-grade voltage stabilizer to protect sensitive optical and digital components. |
| 2. Are spare parts for endodontic microscope magnification systems readily available, and what is the expected lead time? | Yes, leading manufacturers (e.g., Zeiss, Global Surgical, and Planmeca) maintain comprehensive spare parts inventories for 2026 models, including ocular lenses, LED illuminators, boom arms, footswitches, and magnification turrets. Distributors should confirm local warehouse availability or regional service hubs. Standard spare parts are typically shipped within 1–3 business days, while custom or discontinued components may require 2–4 weeks. We recommend clinics maintain a service kit with high-wear items (e.g., bulbs, lens caps, adapter rings) to minimize downtime. Distributors should establish inventory agreements with OEMs to ensure consistent supply chain reliability. |
| 3. What does the installation process involve for a new endodontic microscope with digital magnification integration? | Installation of 2026 endodontic microscopes includes site evaluation, mechanical mounting (ceiling, floor, or wall), electrical connection, optical alignment, and digital calibration. Most systems now support plug-and-play integration with clinic imaging software (e.g., DICOM, intraoral scanners). Certified biomedical technicians or OEM-trained engineers perform installations, typically within 4–6 hours. Pre-installation requirements include a stable mounting surface, dedicated power outlet, and (for digital models) network connectivity. Remote calibration support via AR-assisted tools is now standard. Post-installation training on magnification controls, focus adjustment, and digital capture is included in all premium packages. |
| 4. What warranty coverage is standard for endodontic microscope magnification systems in 2026? | As of 2026, the industry standard is a 3-year comprehensive warranty covering optical components, mechanical articulation, LED illumination, and digital imaging modules. This includes parts, labor, and on-site service for defects in materials or workmanship. Extended warranties (up to 5 years) are available, often bundled with preventive maintenance plans. Note: Damage from improper handling, power surges, or unauthorized modifications is excluded. Distributors should verify warranty activation procedures and ensure clinics register their units within 30 days of installation to maintain full coverage. |
| 5. How are software updates for digital magnification and imaging handled under warranty and post-purchase support? | Software updates for digital magnification, image enhancement algorithms, and integration with practice management systems are provided free of charge under the warranty period and often beyond via manufacturer subscription portals. In 2026, OTA (Over-the-Air) updates are standard, deployable remotely with clinic approval. Updates improve autofocus precision, color rendering, and compatibility with new imaging standards. While hardware-related support is warranty-covered, advanced AI-driven analytics (e.g., pulp cavity detection) may require optional software licensing. Distributors should ensure clients have secure internet access for update deployment and maintain service contracts for continuous technical support. |
Need a Quote for Endo Microscope Magnification?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


