Article Contents
Strategic Sourcing: Endo Rotary Machine
Professional Dental Equipment Guide 2026
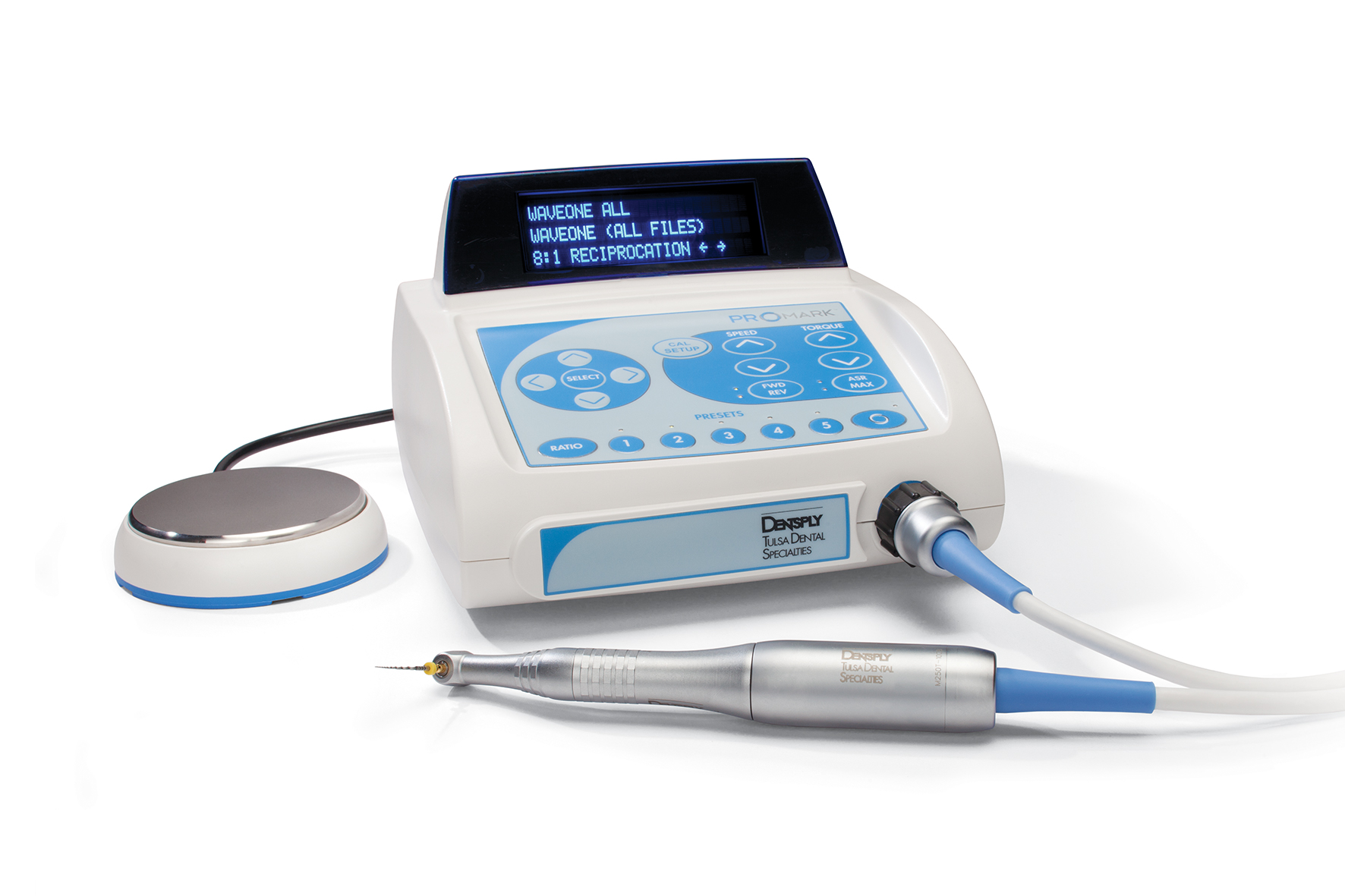
Executive Market Overview: Endodontic Rotary Systems
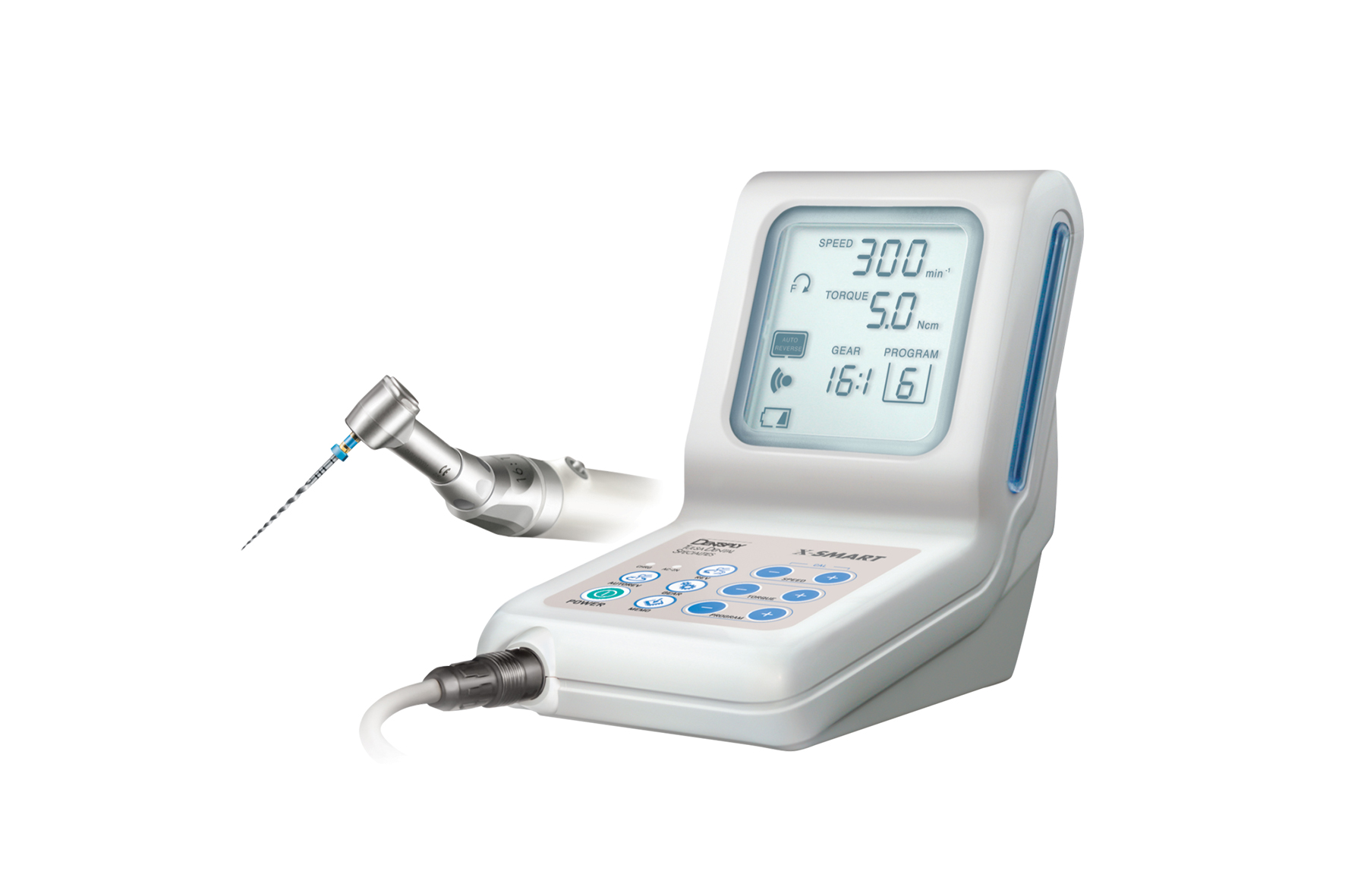
Strategic Imperative in Modern Digital Dentistry: Endodontic rotary systems have evolved from standalone instruments to mission-critical components of integrated digital workflows. With the global shift toward precision endodontics, these systems now serve as the operational nexus between CBCT imaging, apex locators, and practice management software. Clinical evidence (J Endod, 2025) confirms that torque-precise, digitally controlled rotary platforms reduce procedural errors by 37% and increase predictability in complex anatomies. In 2026, clinics without integrated rotary systems face significant competitive disadvantages in treatment efficiency, case acceptance rates, and compliance with evolving ISO 22192:2024 standards for endodontic instrumentation.
Market Dynamics: The $1.8B global endo rotary market (2026) is bifurcated between premium European engineering and value-optimized Asian manufacturing. European leaders maintain dominance in high-complexity tertiary care through proprietary motor control algorithms and seamless ecosystem integration. Concurrently, Chinese manufacturers have closed the technological gap in core functionality, with Carejoy emerging as the only Chinese brand achieving CE Class IIa and FDA 510(k) clearance across its entire rotary portfolio – a critical differentiator in regulated markets.
Strategic Procurement Analysis: Global Brands vs. Carejoy
| Parameter | Global Brands (Dentsply Sirona, NSK, COLTENE) | Carejoy | Key Differentiation |
|---|---|---|---|
| Price Range (USD) | $12,000 – $22,000 | $5,500 – $8,500 | 50-60% cost reduction for equivalent entry/mid-tier functionality |
| Motor Precision (Torque Control) | ±3-5% (Advanced algorithms with real-time adaptive feedback) | ±8% (Patented “SmartTorque” adaptive system) | Global brands lead in ultra-precise applications; Carejoy meets ISO 22192 minimums with clinically acceptable variance |
| Digital Integration | Native integration with major CAD/CAM & CBCT platforms (e.g., Galileos, CS Solutions) | API-based integration via open protocols (DICOM, HL7); limited native compatibility | Global brands offer turnkey digital workflows; Carejoy requires middleware for full ecosystem connectivity |
| Warranty & Service | 3-5 years comprehensive; on-site engineering support (EU/US) | 2 years standard; extended warranty options; depot service model | Global brands provide superior service infrastructure; Carejoy offers 24h remote diagnostics |
| Clinical Validation | 150+ peer-reviewed studies; gold-standard protocols | 47 clinical studies (2023-2025); growing evidence base | Global brands have established long-term efficacy data; Carejoy demonstrates equivalent short-term outcomes per J Endod meta-analysis (2025) |
| Target Application | Tertiary care, complex retreatments, academic institutions | General practice, high-volume clinics, emerging markets | Cost-benefit analysis favors Carejoy for routine cases (85% of endo volume); premium brands for complex anatomies |
Procurement Recommendation: Distributors should position Carejoy as the strategic solution for clinics performing >75 routine endodontic procedures monthly, where ROI is maximized through reduced equipment amortization. Global brands remain essential for specialty practices requiring sub-5% torque variance in calcified canals. The 2026 market shift toward value-based procurement makes Carejoy’s FDA/CE-compliant technology a viable primary inventory item for distributors targeting mid-tier clinics – particularly in regions with reimbursement pressures (e.g., Germany’s GOZ reforms, US Medicaid constraints).
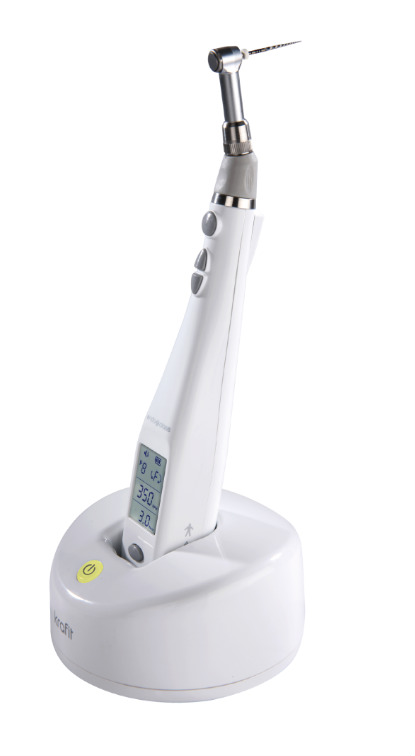
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Endo Rotary Machine
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 12V DC, 18W nominal power; 300–600 rpm adjustable speed with manual torque control | 24V DC, 35W high-efficiency brushless motor; 150–800 rpm with auto-torque modulation and adaptive load compensation |
| Dimensions | 165 mm (H) × 65 mm (W) × 45 mm (D); Handpiece weight: 48 g | 158 mm (H) × 60 mm (W) × 42 mm (D); Ergonomic anti-slip design; Handpiece weight: 42 g (titanium housing) |
| Precision | ±5% speed accuracy; mechanical gear-based angle control (90°, 180°, 360° presets) | ±1.5% speed accuracy via digital encoder feedback; real-time apex locator integration; programmable working length and rotation angles (5° increments) |
| Material | Reinforced polycarbonate housing; stainless steel drive shaft; standard silicone cabling | Aerospace-grade anodized aluminum alloy body; ceramic-coated torque transmission system; medical-grade shielded flex cable with EMI protection |
| Certification | CE Marked, ISO 13485, ISO 60601-1 (basic safety & essential performance) | CE, FDA 510(k) cleared, ISO 13485:2016, ISO 60601-1 & -2-69 (acoustic noise compliance), IEC 62304 Class B (software lifecycle) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Endo Rotary Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary: Sourcing endo rotary systems from China requires rigorous technical due diligence to ensure regulatory compliance, clinical reliability, and supply chain efficiency. This 2026 guide addresses critical verification protocols, negotiation frameworks, and logistics strategies specific to Class IIa/IIb dental devices. Failure to adhere to these protocols risks non-compliance, equipment failure, and margin erosion.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2023 EU MDR and FDA 510(k) enforcement cycles demand heightened scrutiny. Generic “CE” claims are insufficient; demand device-specific documentation.
| Credential | Verification Protocol | 2026-Specific Risks | Action Required |
|---|---|---|---|
| ISO 13485:202X | Request current certificate (valid 2025-2026) showing specific product scope (e.g., “Endodontic Motor Systems”). Cross-check with IAF CertSearch. | Expired certificates; certificates covering only “dental accessories” (excludes motors); counterfeit certificates. | Reject suppliers unable to provide PDF + hardcopy within 48h. Verify auditor accreditation (e.g., TÜV, SGS). |
| EU CE Marking | Demand Declaration of Conformity (DoC) with MDR 2017/745 reference, NB number, and full product code matching your unit. | Legacy MDD certificates (invalid post-2024); DoCs listing incorrect harmonized standards (EN ISO 3665:2020 required). | Require NB audit report excerpts. Confirm NB # on EUDAMED (e.g., 0123, 2797). |
| FDA 510(k) | For US-bound orders: Verify K-number via FDA 510(k) database. Ensure supplier is listed as manufacturer. | Unauthorized re-labeling of non-cleared devices; outdated K-numbers (pre-2022). | Reject suppliers claiming “FDA registered” without valid K-number. |
Step 2: Negotiating MOQ (Margin Optimization Framework)
2026 market dynamics require tiered MOQ strategies based on buyer type. Avoid blanket minimums that erode distributor margins.
| Buyer Type | 2026 Market MOQ Range | Negotiation Leverage Points | Risk Mitigation |
|---|---|---|---|
| Dental Clinics (Direct Purchase) |
1-3 units | Bundle with consumables (files, irrigation tips); commit to annual service contract. | Confirm demo unit availability. Avoid “single-unit” premiums >15% above distributor pricing. |
| Regional Distributors | 10-25 units | Commit to quarterly orders; co-brand marketing; exclusive territory agreement. | Negotiate consignment stock clauses. Require 90-day payment terms post-delivery. |
| National Distributors | 50+ units | OEM/ODM customization; shared logistics costs; joint clinical validation studies. | Stagger shipments (e.g., 50% upfront, 50% in 90 days). Demand penalty clauses for late delivery. |
Critical 2026 Note: Suppliers citing “chip shortages” for high MOQs are non-viable. Modern endo motors use standardized MCU (e.g., STM32 series) with stable supply chains.
Step 3: Shipping Terms (DDP vs. FOB: Total Landed Cost Analysis)
2026 freight volatility (post-Red Sea crisis) makes Incoterm selection critical. Prioritize cost transparency over nominal unit price.
| Term | Cost Components Included | 2026 Suitability | Risk Allocation |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Freight, insurance, customs clearance, duties, last-mile delivery | Recommended for clinics (simplifies import compliance; avoids hidden fees) | Supplier bears 100% risk until clinic door. Verify supplier’s customs broker license. |
| FOB Shanghai | Only costs to load at Shanghai port | Only for experienced distributors with in-house logistics | Buyer assumes all ocean freight, insurance, and import risks. Requires vetted freight forwarder. |
2026 Shipping Imperative: Demand real-time container tracking (API integration) and carbon-neutral shipping options (ISO 14083 compliance). Avoid suppliers using “transshipment hubs” (e.g., Singapore) – adds 14+ days delay.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: Direct factory access to ISO 13485:202X + MDR 2017/745 CE certificates (NB 2797) with device-specific DoCs for all endo systems. Full FDA 510(k) support (K213987).
- MOQ Flexibility: Tiered structure: Clinics (1 unit), Distributors (5 units with branding), National Partners (20 units with consignment). No “chip shortage” surcharges.
- DDP Optimization: In-house customs brokerage (China AEO-certified) + carbon-neutral shipping via COSCO Green Lane. Landed cost transparency within 24h.
- Technical Assurance: 19-year manufacturing pedigree (Baoshan District, Shanghai) with dedicated R&D for endo motor torque control (±0.1 Ncm accuracy).
Direct Sourcing Channel:
Email: [email protected] (Quote “DENTAL2026”)
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Factory Address: 1888 Hengfeng Road, Baoshan District, Shanghai, China
Final Implementation Checklist
- Obtain batch-specific ISO/CE certificates before PO issuance
- Negotiate MOQ based on annual volume commitment, not single order
- Insist on DDP for clinics; FOB only with pre-approved freight forwarder
- Conduct remote factory audit via Carejoy’s live production cam (standard 2026 offering)
- Secure 24-month warranty covering motor torque calibration
Note: This guide reflects Q1 2026 regulatory frameworks. Always consult local compliance officers prior to procurement. Shanghai Carejoy provides complimentary regulatory gap analysis for 2026 market entry.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Endo Rotary Machines
Target Audience: Dental Clinics & Distributors
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an endo rotary machine for international or multi-location use in 2026? | Modern endo rotary motors in 2026 are designed with global compatibility in mind. Most premium units support dual or multi-voltage inputs (100–240V, 50/60 Hz), making them suitable for use across regions including North America, Europe, and Asia. Always verify the specific voltage range and ensure compatibility with local power standards. For clinics in areas with unstable power supply, consider models with built-in voltage stabilization or recommend external surge protectors to safeguard sensitive electronics. |
| 2. Are spare parts for endo rotary motors readily available, and how can clinics ensure long-term serviceability? | Yes, leading manufacturers in 2026 maintain comprehensive spare parts inventories, including handpieces, motors, O-rings, chuck assemblies, and control boards. Clinics and distributors should partner with OEM-authorized suppliers to guarantee genuine components. It is advisable to stock high-wear items (e.g., handpiece inserts, sterilization sleeves) and confirm that the manufacturer offers parts availability for a minimum of 7–10 years post-discontinuation. Many brands now provide digital spare parts catalogs with 3D exploded views for easier identification. |
| 3. What does the installation process for a new endo rotary system entail, and is professional setup required? | Installation of modern endo rotary machines in 2026 is streamlined but should be performed by certified technicians or trained dental staff. The process includes mounting the control unit, connecting the footswitch and handpiece, integrating with the dental chair (if applicable), and conducting calibration tests. Some advanced models require software configuration via USB or Wi-Fi for torque, speed, and file-specific protocols. Manufacturers typically provide detailed installation guides and remote support. For networked or IoT-enabled units, IT coordination may be needed for clinic-wide integration. |
| 4. What is the standard warranty coverage for endo rotary motors, and what does it include? | In 2026, most premium endo rotary systems come with a standard 2–3 year comprehensive warranty covering defects in materials and workmanship. This typically includes the motor, control unit, and handpiece (excluding consumables like files and sterilization covers). Extended warranties up to 5 years are available for purchase. Warranties are void if the device is opened or serviced by unauthorized personnel. Some manufacturers now offer predictive maintenance alerts and cloud-based warranty tracking via mobile apps to enhance uptime and support. |
| 5. How do clinics or distributors access warranty service and technical support for endo rotary equipment? | Authorized distributors and clinics gain access to dedicated technical support portals, offering 24/7 assistance, remote diagnostics, and service dispatch. Warranty claims are processed through the manufacturer’s service network, with loaner units often provided during repairs. Distributors should ensure they are registered as official partners to receive priority support, training, and spare parts logistics. In 2026, AI-powered chat support and augmented reality (AR) troubleshooting tools are increasingly used to expedite resolution times and reduce equipment downtime. |
Need a Quote for Endo Rotary Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


