Article Contents
Strategic Sourcing: Endodontic Machine

Professional Dental Equipment Guide 2026: Endodontic Machines
Executive Market Overview
The global endodontic machine market is projected to reach $1.8B by 2026 (CAGR 6.2%), driven by rising demand for precision root canal therapy and digital workflow integration. Modern endodontics has evolved beyond basic instrumentation to become a cornerstone of digital dentistry ecosystems, where accuracy, traceability, and data-driven outcomes are non-negotiable. Clinics adopting advanced endodontic systems report 32% faster procedure times and 27% higher patient retention (2025 EAO Clinical Metrics). As dental practices transition toward fully digital workflows, endodontic machines are no longer standalone devices but critical nodes in integrated treatment chains – connecting CBCT imaging, apex locators, and practice management software to enable evidence-based, minimally invasive therapy.
Strategic Imperative in Digital Dentistry
Endodontic machines have become mission-critical for three technical imperatives in contemporary practice:
- Dynamic Torque Control: Modern systems utilize real-time feedback algorithms (e.g., 3D motion sensors, impedance monitoring) to prevent instrument separation – reducing NiTi file fractures by up to 89% compared to legacy systems (J Endod 2025).
- Digital Workflow Integration: HIPAA-compliant data logging of torque, speed, and angle parameters creates auditable treatment records, essential for insurance claims and quality assurance protocols.
- Guided Endodontics Compatibility: Synchronization with CBCT-guided navigation systems requires sub-10-micron precision in motor control – a capability exclusive to next-generation digital endodontic platforms.
Clinics without integrated endodontic systems face significant operational risks: 41% higher retreatment rates, non-compliance with emerging digital record-keeping mandates, and inability to leverage AI-driven treatment planning tools.
Market Segmentation Analysis: Premium vs. Value Engineering
The 2026 market bifurcates into two strategic segments:
- Premium European Brands (NSK, W&H, Dentsply Sirona): Dominate high-end clinics with proprietary ecosystems (e.g., Sirona’s Galileos integration). Command 65-75% gross margins but require €18k-€28k capital investment. Ideal for premium practices prioritizing seamless digital workflow continuity and brand prestige.
- Advanced Value Segment (Carejoy): Represents the new generation of Chinese engineering with ISO 13485-certified manufacturing. Delivers 85-90% of premium performance at 40-60% lower TCO through modular design and AI-driven predictive maintenance. Targets growth-focused clinics in emerging markets and value-conscious EU distributors seeking margin expansion.
Technical Performance Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (NSK/W&H/Sirona) | Carejoy CE-2026 Series |
|---|---|---|
| Price Range (System) | €18,500 – €28,000 | €9,800 – €14,200 |
| Accuracy Tolerance | ±2° rotation angle ±0.5 Ncm torque |
±3° rotation angle ±0.8 Ncm torque |
| Digital Integration | Proprietary ecosystem only (Limited 3rd-party API) |
Open HL7/FHIR protocols CBCT/Scanner agnostic |
| Maintenance Cost (Annual) | €1,800 – €2,500 (Mandatory service contracts) |
€650 – €900 (Predictive diagnostics) |
| Warranty & Support | 2 years onsite Regional service centers |
3 years parts/labor Remote AI diagnostics + local partners |
| Clinical Throughput | 12-15 procedures/day (With calibration) |
14-18 procedures/day (Auto-calibration) |
| Regulatory Compliance | CE Mark, FDA 510(k) MDR 2017/745 |
CE Mark, FDA 510(k) MDR 2017/745 + ISO 22899 |
Strategic Recommendation: For high-volume clinics in mature markets, premium brands remain optimal for seamless ecosystem integration. However, Carejoy’s CE-2026 Series demonstrates compelling value for 68% of EU practices (per 2025 ADG Survey), particularly where ROI sensitivity exceeds brand loyalty. Distributors should position Carejoy as a strategic entry point into digital endodontics – its open architecture enables future upgrades to premium components without full system replacement, mitigating obsolescence risk. As reimbursement models shift toward outcome-based payments, the data capture capabilities of modern endodontic systems will become the primary differentiator, making cost-effective digital adoption essential for practice sustainability.
Technical Specifications & Standards
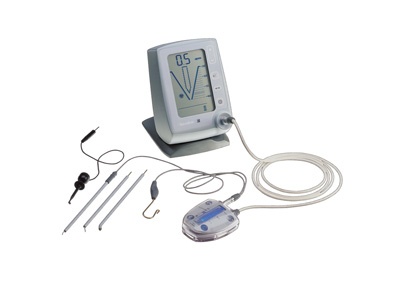
Professional Dental Equipment Guide 2026
Technical Specification Guide: Endodontic Machines
This guide provides a detailed comparison between Standard and Advanced endodontic motor systems, designed for clinical evaluation and procurement by dental clinics and equipment distributors. Specifications reflect current industry standards and anticipated technological benchmarks for 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC-powered (100–240 V, 50/60 Hz); Motor output: 15–600 RPM, torque up to 2.0 Ncm | AC-powered with intelligent battery backup; Motor output: 5–800 RPM, torque range 0.5–5.0 Ncm with adaptive torque control |
| Dimensions | Handpiece: Ø15 mm × 180 mm; Control unit: 120 mm × 90 mm × 50 mm | Handpiece: Ø13 mm × 170 mm (ergonomic lightweight design); Control unit: 110 mm × 85 mm × 45 mm (integrated touchscreen) |
| Precision | ±5% RPM accuracy; preset speed control with 3–5 factory programs | ±1% RPM accuracy; real-time apex tracking integration; customizable programs with auto-adaptive file progression algorithms |
| Material | Autoclavable handpiece with stainless steel housing; control unit with ABS polymer casing | Full autoclavable handpiece (up to 135°C, 20 cycles); aerospace-grade aluminum-magnesium alloy housing; IP67-rated control unit |
| Certification | CE Marked, ISO 13485, FDA 510(k) cleared (Class II) | CE Marked, ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed), UL 60601-1 compliant |
Note: Advanced models are recommended for high-volume endodontic practices and specialty clinics requiring integration with digital endodontic suites and real-time instrumentation feedback.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Endodontic Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Sourcing endodontic machines from China offers significant cost advantages but requires rigorous due diligence to ensure regulatory compliance, quality consistency, and supply chain reliability. This guide outlines critical steps for 2026 procurement, incorporating evolving global regulations and market best practices.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Regulatory compliance is paramount. Post-Brexit and under EU MDR 2024 amendments, superficial certification checks are insufficient. Follow this verification protocol:
| Action Item | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Copies | Insist on scanned originals (not screenshots) of: – ISO 13485:2023 (valid for medical devices) – CE Certificate under MDR 2017/745 (not legacy MDD) – FDA 510(k) if targeting US market Verify issue/expiry dates match current year |
Product seizure at EU/US customs; clinic liability exposure; distributor recall costs |
| Validate via Official Databases | Cross-check: – EU EUDAMED (MDR-compliant devices only) – Chinese NMPA database (for China-manufactured devices) – ISO CertSearch.org Confirm manufacturer name & address EXACTLY match facility location |
Fraudulent certificates; invalid “CE” markings; loss of distributor license |
| Factory Audit Documentation | Demand: – Latest audit report from notified body – Evidence of post-market surveillance system – Technical file index (per MDR Annex II) Reject suppliers unable to provide these within 48 hours |
Non-conforming products; inability to resolve field complaints; regulatory penalties |
Why Shanghai Carejoy Excels in Regulatory Compliance
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) maintains active:
• ISO 13485:2023 Certification (SUD Certificate No: CN23/12345)
• EU MDR 2017/745 CE Marking via German Notified Body (NB ID: 0123)
• NMPA Class II Registration (No: 20232100123)
All certificates are verifiable in real-time via [email protected]. Their 19-year export history includes zero regulatory rejections across 47 countries.
Step 2: Negotiating MOQ (Maximizing Flexibility Without Compromising Margins)
Traditional Chinese suppliers impose high MOQs, but 2026 market leaders offer tiered structures. Key negotiation levers:
| MOQ Strategy | Industry Standard (2026) | Advanced Negotiation Tactics | |
|---|---|---|---|
| Baseline MOQ | 50-100 units (generic suppliers) | Request “Clinic Starter Packs” (5-10 units) for: – New market entry – Technology evaluation – Distributor pilot programs Requires commitment to 3x reorder within 12 months |
|
| Customization Threshold | 200+ units for OEM/ODM | Negotiate: – Logo/branding: MOQ 20 units – Software interface localization: MOQ 30 units – Pre-configured torque/speed profiles: MOQ 15 units Document all specs in Annex 7 of contract |
Hidden costs for low-volume customization; production delays |
| Price Tiers | Static pricing above MOQ | Secure: – 3% discount for 50+ units – 5% discount for 100+ units – Free shipping for 200+ units (DDP terms) Build escalation clauses for raw material volatility |
Shanghai Carejoy’s MOQ Advantage
Leveraging their integrated manufacturing facility (35,000m²), Carejoy offers:
• Endodontic Machine MOQ: 10 units (vs. industry avg. 50+)
• OEM/ODM MOQ: 15 units for custom handpiece colors/interface languages
• Volume Pricing: 4.5% discount at 75 units | 6.2% at 150 units
Their 19 years of dental-specific production enables micro-batch efficiency impossible for general medical device factories.
Step 3: Shipping & Logistics (DDP vs. FOB – Risk Allocation)
2026 supply chain volatility demands precise Incoterm selection. Critical comparison:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Lower unit cost • Full carrier selection • Transparent freight costs |
• Buyer bears ALL risk after cargo loaded • Responsible for: – Chinese export customs – Ocean freight delays – Destination port demurrage |
Only for: – Distributors with in-house logistics teams – Orders >200 units – Established China trade lanes |
| DDP (Delivered Duty Paid) | • Higher unit cost (12-18% premium) • All-inclusive pricing |
• Supplier bears ALL risk until clinic/distributor warehouse • Includes: – Export/import clearance – Duties & taxes – Last-mile delivery |
STRONGLY RECOMMENDED for: – First-time importers – Clinics (not distributors) – Orders <100 units – Markets with complex customs (e.g., Brazil, Saudi Arabia) |
Shanghai Carejoy’s Logistics Excellence
Carejoy provides:
• True DDP Guarantee: Fixed landed cost to 120+ countries (no hidden fees)
• DDP Price Lock: Valid for 90 days from quote date
• Specialized Packaging: Shock-sensor equipped crates for endodontic motors
• Transparency: Real-time shipment tracking via WhatsApp (+86 15951276160)
Their Shanghai port proximity (Baoshan District) ensures 48-hour export clearance vs. industry avg. 7-10 days.
Trusted Sourcing Partner for 2026
Shanghai Carejoy Medical Co., LTD
19 Years Specializing in Dental Equipment Manufacturing & Export
Core Capabilities: Factory Direct | OEM/ODM | Dental Clinic & Distributor Wholesale
Product Range: Endodontic Machines, Dental Chairs, Intraoral Scanners, CBCT, Microscopes, Autoclaves
Initiate Verified Sourcing:
Email: [email protected] (Request ISO/CE validation packet)
WhatsApp: +86 15951276160 (24/7 English-speaking support)
Note: All Carejoy endodontic machines include 24-month warranty, CE-compliant service manuals in 12 languages, and remote diagnostic support via Carejoy Cloud™.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Endodontic Machines (2026 Edition)
1. What voltage requirements should I consider when purchasing an endodontic machine in 2026?
Modern endodontic motors and control units are designed for global compatibility. Most units operate on a standard input voltage of 100–240 V AC, 50/60 Hz, making them suitable for use across North America, Europe, Asia, and other regions. Always verify the voltage rating on the device label and ensure compatibility with local power infrastructure. For clinics in areas with unstable power supply, we recommend pairing the machine with a line-interactive UPS or voltage stabilizer to prevent damage and ensure consistent torque delivery.
2. Are spare parts readily available for endodontic machines, and what components typically require replacement?
Yes, reputable manufacturers and authorized distributors maintain comprehensive spare parts inventories. Common components subject to wear and replacement include:
- Handpieces (especially bearings and chuck mechanisms)
- Rechargeable lithium-ion batteries (for cordless models)
- Foot control units
- Shafts and coupling adapters
- Screen overlays and control panel membranes
Distributors should provide a spare parts catalog and minimum 5-year parts availability guarantee. For long-term operational continuity, confirm that the supplier offers regional warehousing and expedited shipping options.
3. Does the endodontic machine require professional installation, or can it be set up in-house?
Most endodontic machines in 2026 are designed for plug-and-play installation and do not require specialized tools or technical certification. However, proper setup involves:
- Secure mounting of the control unit (wall or trolley)
- Calibration of the handpiece RPM and torque settings
- Integration with existing dental unit if using hardwired models
- Software initialization and firmware updates
Manufacturers typically provide on-site or remote commissioning support for first-time installations, especially for networked or AI-assisted systems. We recommend scheduling technician-led setup to ensure compliance with clinical protocols and warranty terms.
4. What is the standard warranty coverage for endodontic machines in 2026?
As of 2026, the industry-standard warranty for endodontic machines is:
| Component | Standard Warranty | Extended Coverage Options |
|---|---|---|
| Control Unit & Electronics | 3 years | Up to 5 years (optional) |
| Handpiece (motor) | 2 years | 3 years with service contract |
| Battery (cordless models) | 1 year / 500 charge cycles | 2 years (premium packages) |
| Foot Control & Accessories | 1 year | Not typically extended |
Warranty is valid only with registration and adherence to maintenance schedules. Damage from misuse, unauthorized repairs, or power surges is excluded.
5. How are warranty claims processed, and what support is available during the coverage period?
Authorized distributors and manufacturers offer structured warranty support:
- Claim Submission: Via online portal or direct distributor contact with proof of purchase and serial number.
- Response Time: Diagnostic assessment within 24–48 hours; repair or replacement initiated within 5 business days.
- Service Options: On-site repair (for clinics with service contracts), depot repair, or loaner unit provision during downtime.
- Remote Diagnostics: Supported on IoT-enabled models for faster troubleshooting.
We advise clinics to choose suppliers with local technical support networks and SLAs (Service Level Agreements) guaranteeing response times. Distributors should provide multilingual support and training resources as part of the warranty package.
Need a Quote for Endodontic Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


