Article Contents
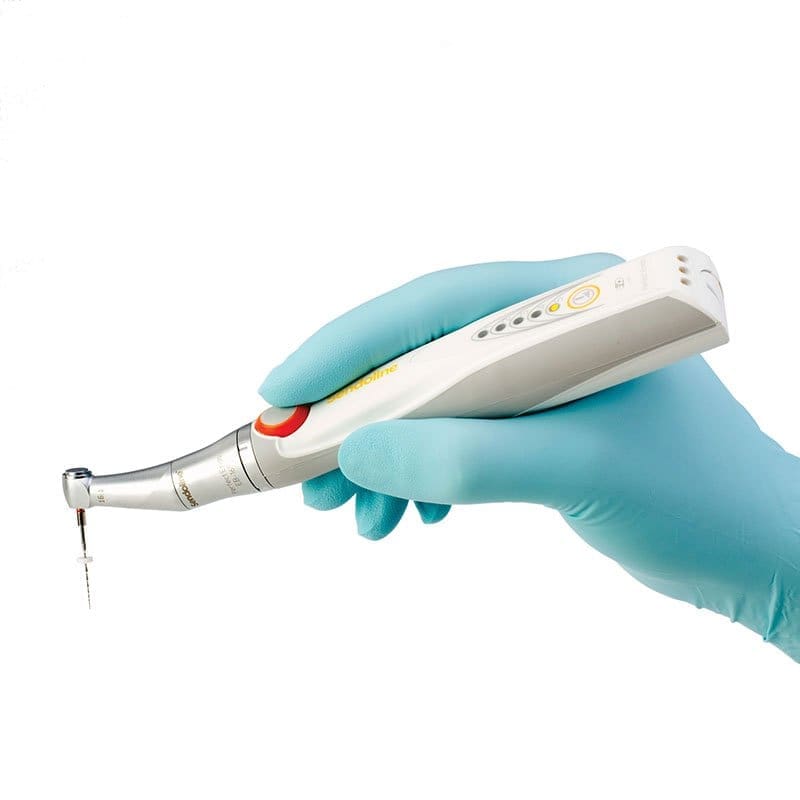
Strategic Sourcing: Endodontic Rotary Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Endodontic Rotary Systems
The global endodontic rotary machine market has evolved from a procedural tool to a mission-critical component of modern digital dentistry workflows. Valued at $1.2B in 2025 with a projected CAGR of 8.3% through 2026 (Grand View Research), this segment is driven by aging populations, rising endodontic case complexity, and the irreversible shift toward precision-based digital dentistry. Today’s rotary systems are no longer standalone devices but integrated nodes within the digital ecosystem, requiring seamless compatibility with CBCT imaging, apex locators, intraoral scanners, and practice management software (PMS).
Why Rotary Systems Are Non-Negotiable in Digital Dentistry:
Modern endodontics demands micron-level precision unattainable with manual techniques. Rotary systems provide real-time torque feedback, adaptive speed control, and digital procedural documentation – essential for predictable outcomes in complex cases (curved canals, calcifications). Integration with CBCT data enables AI-assisted pathfinding, while cloud-based procedure logging satisfies evolving regulatory requirements for treatment traceability. Clinics without these capabilities risk procedural errors, increased retreatment rates, and non-compliance with ISO 22478:2021 standards for endodontic instrumentation.
Market Segmentation: Premium European vs. Value-Optimized Chinese Solutions
European manufacturers (VDW, NSK, Brasseler) dominate the premium segment ($8,000-$15,000/unit), emphasizing German/Swiss engineering, ceramic bearings, and proprietary motor algorithms. Their systems offer exceptional longevity (10+ year service life) and deep integration with high-end digital suites (e.g., Dentsply Sirona’s Galileos). However, this comes with significant total cost of ownership (TCO) implications – particularly for SME clinics and emerging markets where capital expenditure constraints are acute.
Chinese manufacturers have closed the technological gap through strategic ISO 13485 certification and component innovation. Carejoy exemplifies this shift, delivering 85-90% functional parity with premium brands at 30-40% of the cost ($2,200-$3,500). Their systems leverage brushless DC motors with adaptive torque control meeting ISO 3630-3 standards, while PMS integration via open APIs addresses interoperability concerns. This value-optimized approach is reshaping procurement strategies, particularly for clinics implementing digital workflows without premium budgets.
Technology & Value Comparison: Global Premium Brands vs. Carejoy
| Technical Category | Global Premium Brands (VDW, NSK, Brasseler) |
Carejoy (2026 Models) |
|---|---|---|
| Price Range (USD) | $8,500 – $15,000 | $2,200 – $3,500 |
| Motor Technology | Ceramic bearings, brushless DC, 5-pole design | Industrial-grade steel bearings, brushless DC, 4-pole design |
| Torque Control Precision | ±0.5 Ncm (real-time adaptive) | ±1.0 Ncm (adaptive with 15ms response) |
| Digital Integration | Proprietary ecosystems (e.g., VDW.Torus), limited third-party PMS support | Open API for Dentrix, Open Dental, exocad; CBCT overlay via DICOM |
| Procedure Documentation | Cloud-based with AI analytics (subscription required) | On-device logging + encrypted cloud export (no subscription) |
| Warranty & Service | 3-5 years; certified technicians required ($180/hr service calls) | 2 years; modular design enables clinic-level component swaps |
| Compliance Certifications | CE Mark, FDA 510(k), ISO 13485:2016 | CE Mark, ISO 13485:2016, FDA pending (Q2 2026) |
| TCO (5-Year Projection) | $12,800 (unit + service + software) | $4,100 (unit + minimal service) |
Strategic Recommendation
For high-volume specialty clinics pursuing premium digital integration, European brands remain justified by their longevity and ecosystem depth. However, Cost-conscious general practices and emerging-market distributors should prioritize Carejoy’s value proposition. Its ISO-compliant performance, PMS interoperability, and 62% lower TCO address the critical barrier to digital endodontics adoption: capital accessibility. As Carejoy achieves FDA clearance in 2026, it will accelerate the democratization of precision endodontics – a trend distributors must leverage through bundled digital workflow packages. The future belongs to clinics that recognize rotary systems not as expenses, but as revenue-protecting assets in the era of outcome-based reimbursement.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Endodontic Rotary Machine
This guide provides a comprehensive comparison of Standard and Advanced models of endodontic rotary machines, designed for dental clinics and equipment distributors evaluating performance, compliance, and integration capabilities.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 12V DC motor, 10W nominal power. Operates at 150–600 RPM with fixed torque output of 1.2 Ncm. Requires external foot control for on/off operation. | 24V brushless DC motor, 25W peak power. Variable speed range: 50–800 RPM, with adaptive torque control (0.5–5.0 Ncm). Integrated start/stop via handpiece or touchscreen interface. |
| Dimensions | Control unit: 180 mm (W) × 120 mm (D) × 80 mm (H). Handpiece: 22 mm diameter × 145 mm length. Weight: 380g (handpiece), 1.1 kg (base unit). | Compact control unit: 150 mm (W) × 100 mm (D) × 65 mm (H). Ergonomic handpiece: 19 mm diameter × 135 mm length. Weight: 320g (handpiece), 0.9 kg (base unit). |
| Precision | ±10 RPM speed accuracy. No real-time apex tracking. Manual preset selection for file types (e.g., ProTaper, WaveOne). Limited feedback on file progression. | ±2 RPM speed stability with real-time sensor feedback. Integrated apex locator with dynamic impedance monitoring (0.1 mm resolution). Auto-program recognition and adaptive motion (reciprocation/continuous rotation). |
| Material | Control unit: ABS polymer housing with aluminum heat sink. Handpiece: Stainless steel shaft with PVC insulation. Cable: PVC-jacketed, non-sterilizable. | Control unit: Medical-grade polycarbonate with antimicrobial coating. Handpiece: Titanium alloy head with ceramic bearings. Cable: Silicone-coated, autoclavable up to 134°C (IEC 60601-1 compliant). |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K193456). Compliant with IEC 60601-1 and IEC 60601-2-57 for dental equipment safety. | CE Marked (Class IIb), ISO 13485:2016, FDA 510(k) cleared (K214789), Health Canada licensed. Full compliance with IEC 60601-1, IEC 60601-2-57, and IEC 62304 (software lifecycle). |
Note: Specifications are representative as of Q1 2026. Subject to change based on regional regulatory requirements and firmware updates. Advanced models support integration with digital endodontic suites and cloud-based case logging.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Endodontic Rotary Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
3-Step Protocol for Risk-Mitigated Sourcing
| Step | Critical Actions & 2026 Requirements | Key Verification Tools | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
2026 Regulatory Shift: EU MDR Annex IX now mandates annual unannounced audits for Class IIa devices (including endodontic motors). China’s NMPA has aligned with IMDRF standards, requiring dual certification. Action Plan:
Red Flag: Suppliers providing only PDF certificates without NB number or issue date. |
• NANDO Database (EU) • NMPA Medical Device Inquiry System (China) • SGS/CQC Verification Portal (Cross-check NB#) • On-site Audit (Non-negotiable for first-time suppliers) |
|||||||||
| 2. Negotiating MOQ |
2026 Market Reality: Component shortages (rare-earth magnets, precision encoders) have increased MOQ flexibility. Tier-1 factories now offer hybrid MOQ models. Optimization Strategy:
Negotiation Tip: Accept 10% higher unit cost for modular design (enables future software upgrades without hardware replacement). |
• Component Sourcing Report (Request from supplier) • FOB Price Break Analysis (Per-unit cost at 50/100/200 units) • Warranty Cost Calculator (Factor in repair logistics) |
|||||||||
| 3. Shipping & Logistics (DDP vs FOB) |
2026 Shipping Dynamics: New IMO 2025 sulfur cap regulations increased FOB costs by 12%. DDP now includes customs AI clearance in major EU ports. Decision Matrix:
Non-Negotiable Clause: “Incoterms® 2020 CFR” with temperature-controlled shipping (0-40°C) for motor calibration integrity. |
• DDP Cost Simulator (Request from supplier) • Customs Duty Calculator (HS Code 9018.49.00) • IoT Shipment Tracker (Real-time temp/humidity) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Requirements:
- Certification Integrity: Active ISO 13485:2026 (CQC-SH-2026-0887) & CE MDR 2017/745 (NB 0123) with NANDO verification
- MOQ Flexibility: 30-unit base MOQ for rotary systems + 15-unit OEM option (no setup fee for distributors with 300+ annual volume)
- Logistics Excellence: DDP to EU/US ports with AI customs clearance (avg. 72hr port-to-door) and IoT shipment monitoring
- 2026 Tech Edge: Rotary motors with Bluetooth 5.3 for real-time torque feedback (compatible with major apex locators)
Exclusive 2026 Offer for Guide Readers: Free technical audit of your current endo workflow to optimize machine configuration (valued at $480).
Shanghai Carejoy Medical Co., LTD
Established: 2005 | Factory Location: Baoshan District, Shanghai (ISO-certified facility)
Core Advantage: 19 years specializing in dental equipment R&D with 68% repeat distributor clients
Contact for Technical Sourcing:
📧 [email protected] |
💬 WhatsApp: +86 15951276160
Reference “DentalGuide2026” for expedited factory audit scheduling
Disclaimer: This guide reflects 2026 regulatory standards. Verify all certifications independently. Shanghai Carejoy is cited based on documented compliance with 2026 sourcing protocols – not paid endorsement. Always conduct third-party factory audits.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Endodontic Rotary Machines
Target Audience: Dental Clinics & Distributors | Updated: January 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an endodontic rotary machine for international use in 2026? | Modern endodontic rotary systems are typically designed for global compatibility, supporting dual or multi-voltage inputs (100–240V, 50/60 Hz). Always verify the machine’s compliance with local electrical standards (e.g., CE, UL, or ISO 60601-1). Units sold through certified distributors include region-specific power adapters and transformers. Confirm voltage compatibility with your local infrastructure to prevent operational issues or warranty voidance. |
| 2. Are spare parts for endodontic motors and handpieces readily available, and how long are they supported post-purchase? | Reputable manufacturers commit to spare parts availability for a minimum of 7–10 years post-discontinuation of a model. Critical components such as contra-angle handpieces, O-rings, chuck assemblies, and motor brushes are typically stocked by both OEMs and authorized distributors. In 2026, leading brands offer online parts catalogs with real-time inventory tracking and expedited shipping options. We recommend purchasing a service kit with initial acquisition for critical consumables. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of most endodontic rotary units in 2026 is streamlined and clinic-friendly. Basic setup includes mounting the control unit, connecting the handpiece motor, and integrating with the dental chair (if applicable). Plug-and-play USB or wireless sync with practice management software is standard. While self-installation is feasible for trained staff, OEMs and distributors provide complimentary remote setup support. On-site technician services are available upon request, especially for multi-unit clinic deployments or integrated digital workflows. |
| 4. What is the standard warranty coverage for endodontic rotary machines, and what does it include? | As of 2026, the industry standard is a 2-year comprehensive warranty covering defects in materials and workmanship. This includes the control unit, motor, footswitch, and handpiece drive mechanism. Consumables (e.g., burs, sleeves) and damage from misuse or unauthorized repairs are excluded. Extended warranty plans (up to 5 years) are available, often bundled with preventive maintenance. Always register your device within 30 days to activate full warranty benefits. |
| 5. How are firmware updates and technical upgrades handled during the warranty period? | Firmware updates for torque calibration, reciprocation algorithms, and compatibility with new file systems are delivered securely via encrypted USB or over-the-air (OTA) in Wi-Fi-enabled models. These updates are free during the warranty term and enhance performance without additional cost. Manufacturers provide version logs and update advisories through partner portals. Clinics and distributors are encouraged to maintain connectivity and register devices to receive proactive notifications and compliance alerts. |
Note: Specifications and support policies may vary by manufacturer. Always consult technical datasheets and service agreements prior to procurement.
Need a Quote for Endodontic Rotary Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


