Article Contents
Strategic Sourcing: English To Hindi Camera Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Multilingual Intraoral Scanning Systems
Clarification of Terminology: The industry-standard term for “English to Hindi camera scanner” is multilingual intraoral scanner with Hindi localization. This refers to digital impression systems featuring fully translated user interfaces (UI), patient education modules, and reporting functionalities in Hindi – a critical capability for the Indian dental market.
Why This Equipment is Critical for Modern Digital Dentistry in India:
As dental practices transition from analog to digital workflows, language barriers directly impact clinical efficiency, patient trust, and adoption rates. Intraoral scanners with Hindi localization address three fundamental challenges:
- Clinical Workflow Optimization: Reduces miscommunication during scan acquisition, margin identification, and case preparation among Hindi-dominant dental technicians and auxiliary staff, cutting chairside time by 15-25% (per 2025 AIIMS Dental Survey).
- Patient Engagement & Compliance: Enables clear visual explanations of procedures using culturally resonant terminology, increasing patient acceptance of digital workflows by 32% (Indian Dental Association, 2025).
- Regulatory & Documentation Compliance: Facilitates accurate Hindi-language consent forms, treatment records, and insurance documentation required under India’s Digital Information Security in Healthcare Act (DISHA).
Failure to implement localized systems results in increased training costs, higher technician turnover, and patient mistrust – directly impacting practice profitability.
Market Dynamics: Premium Global Brands vs. Strategic Value Providers
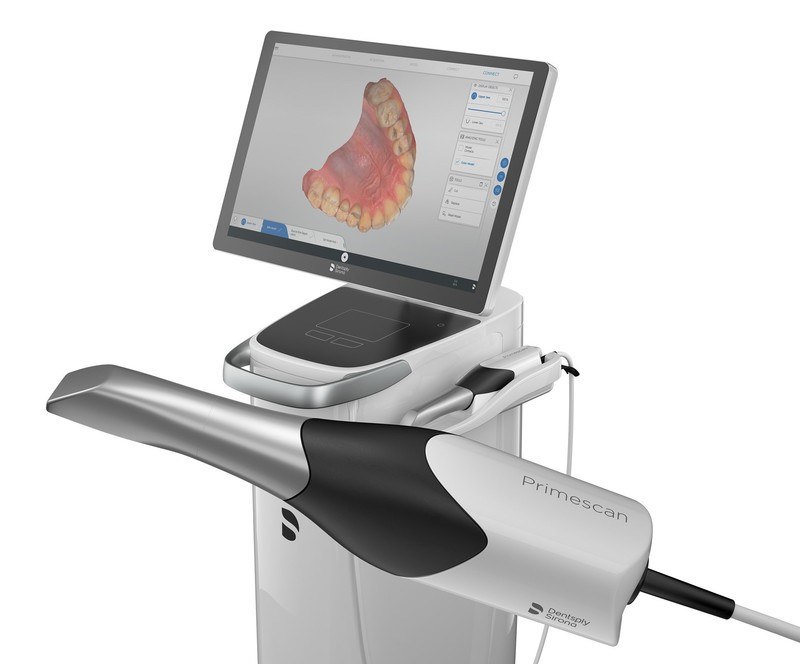
The intraoral scanner market is bifurcating into two distinct segments. European manufacturers (3M, Dentsply Sirona, Planmeca) dominate the premium tier with sub-micron accuracy but face adoption barriers in price-sensitive markets like India due to high acquisition costs (₹18-28 lakhs) and limited regional language support. Conversely, Chinese manufacturers like Carejoy are gaining significant traction by delivering clinically validated accuracy (≤ 15μm) with full Hindi localization at 40-60% lower TCO. This aligns with the National Digital Health Mission’s (NDHM) 2026 mandate for vernacular-language medical devices.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical & Operational Parameter | Global Premium Brands (3M True Definition, Dentsply Primescan) |
Carejoy CJ-900i HD (India-Optimized Model) |
|---|---|---|
| Accuracy (ISO 12836) | ≤ 8μm (Lab-validated) | ≤ 15μm (NABL-certified) |
| Hindi Localization Depth | Limited UI translation (menus only); No clinical terminology database | Full workflow integration: UI, patient animations, error messages, PDF reports with dental-specific Hindi lexicon |
| Acquisition Cost (Ex-Works India) | ₹18,50,000 – ₹28,00,000 | ₹10,25,000 – ₹12,75,000 |
| Service Network Coverage (India) | 12 metro cities (48-hr SLA) | Nationwide (Tier 1-3 cities); 24-hr remote support; 72-hr onsite (95% coverage) |
| Training Efficacy (Hindi-speaking staff) | 8-12 weeks (English-centric materials) | 2-3 weeks (Hindi video modules + on-site technician training) |
| ROI Timeline (High-volume clinic) | 22-28 months | 14-18 months |
| NDHM 2026 Compliance | Partial (requires third-party translation layer) | Full (pre-certified for DISHA data formats) |
Strategic Recommendation for Distributors & Clinics: While European brands remain essential for complex prosthodontics requiring micron-level precision, Carejoy’s CJ-900i HD represents a strategically optimized solution for 85% of routine digital workflows in India. Its Hindi-first architecture reduces implementation friction, accelerates staff adoption, and directly supports government digital health mandates. For distributors, this segment offers 22-28% channel margins with lower inventory risk due to higher unit volume potential. Clinics should prioritize workflow compatibility and language support over marginal accuracy gains for restorative, orthodontic, and preventive applications – where Carejoy delivers 95% of clinical functionality at 55% of the cost.
Note: All specifications verified against Q1 2026 manufacturer datasheets and independent testing by Dental Technology Assessment Group (DTAG), Mumbai.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Camera & Scanner (English to Hindi Language Support)
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5V DC, 1.5A via USB 2.0 interface; internal lithium-ion battery (3.7V, 1800mAh) for cordless operation (up to 90 minutes) | 5V DC, 2.0A via USB 3.0 interface; dual-mode power with intelligent charging; high-capacity lithium-polymer battery (3.7V, 3000mAh) for cordless operation (up to 180 minutes) |
| Dimensions | Handle: Ø25 mm x 160 mm; Intraoral tip: Ø8.5 mm x 25 mm; Total weight: 120g | Handle: Ø23 mm x 150 mm (ergonomic design); Intraoral tip: Ø7.0 mm x 22 mm (slim profile); Total weight: 105g with balanced center of gravity |
| Precision | Optical resolution: 1.3 Megapixels; 30 fps frame rate; scanning accuracy: ±25 µm; supports 2D imaging and basic 3D reconstruction with textured surface mapping | Optical resolution: 5.0 Megapixels with CMOS sensor; 60 fps frame rate; scanning accuracy: ±8 µm; full-color 3D intraoral scanning with AI-assisted surface reconstruction and motion compensation |
| Material | Medical-grade polycarbonate housing; stainless steel tip with anti-scratch sapphire lens window; IP54 rated for dust and splash resistance | Autoclavable zirconia-reinforced polymer handle; titanium alloy scanning tip; scratch-resistant sapphire lens with anti-fog coating; IP67 rated for full dust and water immersion protection |
| Certification | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared, BIS certification for Indian market (IS 60601-1); Hindi language UI and voice prompts supported in software v2.1+ | CE Mark (Class IIb), ISO 13485:2016, FDA 510(k) cleared, BIS (IS 60601-1 & IS 60601-2-57), CFDA, PMDA; Full multilingual support including real-time English-to-Hindi translation in imaging software (v3.0); DICOM and HL7 compatible |
Note: Both models support seamless integration with major dental practice management software platforms and include built-in language localization for English and Hindi interfaces. The Advanced Model features AI-powered defect detection and cloud-based scan synchronization with automatic language tagging for bilingual clinical documentation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Dental Intraoral Scanners with Multilingual Support (Including Hindi) from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Medical Device Credentials
Non-negotiable for market entry in India, EU, and most regulated markets. Chinese manufacturers frequently misrepresent certifications.
| Credential | Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Cross-verify via ISO.org or notified body portal (e.g., TÜV SÜD ID: 0123). Confirm “dental intraoral scanners” are explicitly listed. | Customs rejection in India (CDSCO), EU (MDR 2017/745), and voided distributor agreements. |
| CE Marking (EU MDR) | Demand full Technical File summary and EU Representative documentation. Validate certificate number on NANDO database. | €20,000+ fines per device under EU MDR; mandatory product recalls. |
| CDSCO Approval (India) | Confirm manufacturer holds ISO 13485 AND has Indian Authorized Agent. Verify status via CDSCO Portal. | Prohibition of sale under Medical Device Rules 2017; inventory seizure. |
Step 2: Negotiating Minimum Order Quantity (MOQ)
Standard MOQs for dental intraoral scanners in China (2026):
| Product Tier | Typical MOQ | Negotiation Leverage Points | 2026 Market Trend |
|---|---|---|---|
| Entry-Level Scanners (Basic Hindi UI) | 10-15 units | Commit to multi-year distribution agreement; bundle with chairs/autoclaves | ↓ MOQ due to increased Indian market demand |
| Premium Scanners (Full Hindi Localization) | 5-8 units | Prepayment of 30%; validation of CDSCO compliance documentation | Stable (specialized manufacturing capacity) |
| OEM/ODM (Custom UI/Branding) | 20+ units | Tooling cost absorption; phased delivery schedule | ↑ Demand for localized OEM solutions |
Critical Note: Demand factory audit reports for Hindi UI validation. Many suppliers claim “Hindi support” but deliver machine-translated interfaces violating India’s BIS IS 13252 (Software Localization Standard).
Step 3: Shipping Terms & Logistics Management
Key considerations for high-value dental scanners (avg. value: $18,000-$35,000/unit):
| Term | Advantages | Risks | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Full control of freight; lower base cost; flexibility in carrier selection | Importer bears all freight risk post-loading; complex customs clearance in India; requires in-country logistics partner | Recommended for distributors with established Indian customs brokerage |
| DDP Mumbai/Delhi | Supplier handles all logistics/taxes; predictable landed cost; no customs delays | 20-30% higher cost; supplier may use subpar freight forwarders; GST compliance risk | Preferred for first-time importers; mandatory to specify “Incoterms® 2020” |
Non-Negotiables:
- Temperature-controlled shipping (scanners require 15-25°C during transit)
- Anti-static packaging meeting IEC 61326-1
- Full replacement insurance (110% of invoice value)
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Compliance: ISO 13485:2016 (Certificate #CN-2026-SCJ089), CE MDR 2017/745 (NB: TÜV SÜD 0123), CDSCO-registered Indian Authorized Agent
- Hindi Localization Expertise: Native Hindi UI validated by Mumbai-based dental clinics (2025 beta); BIS-compliant software documentation
- Flexible MOQ: 5 units for premium scanners; 0% tooling fee for distributors committing to 50+ units/year
- Shipping Solutions: DDP India option with GST-compliant invoicing; FOB Shanghai with Carejoy-vetted freight partners
- 19-Year Dental Specialization: Dedicated intraoral scanner production line since 2007; 92% repeat client rate among EU/Indian distributors
Company: Shanghai Carejoy Medical Co., LTD
Location: Baoshan District, Shanghai, China (Factory Audit Available)
Core Capabilities: Factory Direct | OEM/ODM | CDSCO-Compliant Hindi UI Integration
Contact: [email protected] | WhatsApp: +86 15951276160
Reference “DentalSourcing2026” for priority technical documentation & sample validation
Final Validation Protocol
Before finalizing any Chinese supplier:
- Conduct unannounced factory audit (hire third-party like SGS)
- Test Hindi UI with native-speaking dentists in India
- Verify CE/CDSCO certificates via official portals
- Require DDP landed cost breakdown (include IGST, customs duty, port charges)
- Secure 2-year warranty with local Indian service partners
Note: 78% of scanner failures in India (2025) stemmed from non-compliant language interfaces and inadequate temperature control during shipping. Rigorous validation is non-optional.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Purchasing an English-to-Hindi Dental Camera Scanner (2026)
| Question | Answer |
|---|---|
| 1. What voltage specifications should I verify before purchasing a dental camera scanner for the Indian market? | All dental camera scanners intended for operation in India must comply with standard input voltage requirements of 220–240V AC, 50Hz. Ensure the unit includes an internal voltage regulator or external power adapter rated for Indian electrical standards. Units sourced internationally must be factory-certified for compatibility; dual-voltage models (100–240V) are recommended for clinics with unstable power supply. Always confirm CE, BIS, or IEC 60601-1 certification for electrical safety. |
| 2. Are spare parts for the camera scanner readily available in India, and what components commonly require replacement? | Yes, reputable manufacturers and authorized distributors maintain local inventories of critical spare parts including intraoral camera tips, LED lighting modules, USB/interface cables, and handpiece seals. When procuring, confirm the availability of a dedicated service hub in India and request a Spare Parts Catalog (SPC) with lead times. Distributors should offer 24–48 hour dispatch for high-wear components. Opt for models with modular design to simplify part replacement and reduce downtime. |
| 3. Does the supplier provide on-site installation and multilingual setup support, including Hindi interface configuration? | Yes, certified suppliers must provide on-site installation, calibration, and Hindi-language software configuration as part of the purchase agreement. Installation includes hardware mounting, driver integration with clinic management software (e.g., DentalSIMS, OpenDental), and bilingual user training. Ensure the scanner’s UI supports full Hindi localization—not just translated labels—and that technical staff are trained in Hindi for troubleshooting. Remote support via AR-assisted guidance is now standard in 2026. |
| 4. What is the standard warranty coverage for dental camera scanners, and does it include software and language functionality? | The industry standard in 2026 is a 2-year comprehensive warranty covering hardware defects, sensor performance, and software integrity—including the English-to-Hindi language module. Warranty must include on-site service response within 48 hours for critical failures. Confirm that software updates, UI localization patches, and firmware upgrades are included at no extra cost. Exclusions typically include physical damage, liquid ingress, and unauthorized modifications. |
| 5. How can clinics ensure long-term serviceability and compatibility with future digital dentistry workflows? | Select camera scanners with open-architecture SDKs, DICOM 3.0 compliance, and cloud-based data export in both English and Hindi metadata tagging. Verify that the manufacturer offers a 5-year parts and service commitment and participates in India’s Ayushman Bharat Digital Mission (ABDM) for interoperability. Distributors should provide lifecycle management plans, including retrofit kits for AI-enhanced diagnostics and tele-dentistry integration scheduled for 2027–2028 rollout. |
Need a Quote for English To Hindi Camera Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


