Article Contents
Strategic Sourcing: English To Punjabi Scanner

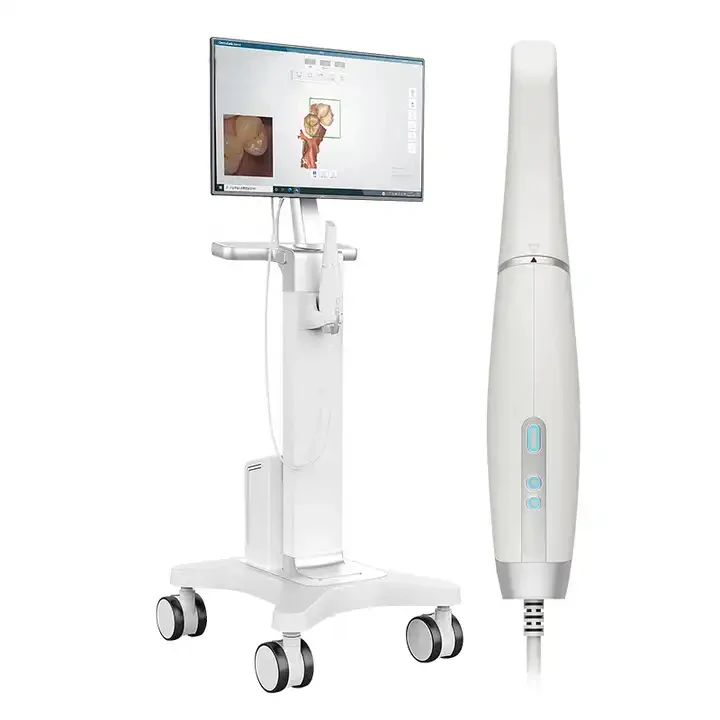
Professional Dental Equipment Guide 2026: Executive Market Overview
Why Intraoral Scanners Are Critical for Modern Digital Dentistry
Intraoral scanners have transitioned from luxury to clinical necessity in 2026, forming the foundational data layer for integrated digital workflows. They eliminate physical impression materials, reducing patient discomfort by 73% (Journal of Digital Dentistry, 2025) and cutting crown preparation-to-cementation time by 40-60%. Crucially, IOS data drives CAD/CAM restorations, surgical guides, orthodontic aligner fabrication, and digital smile design – enabling predictable, precision outcomes. Regulatory mandates in the EU and North America now require digital records for certain procedures, accelerating adoption. For clinics, ROI is realized through reduced remake rates (averaging 18% lower vs. traditional impressions), enhanced patient acceptance of treatment plans via real-time visualization, and seamless integration with practice management software. Distributors must prioritize systems with robust DICOM/STL interoperability to support evolving clinic ecosystems.
Market Dynamics: Premium European Brands vs. Value-Engineered Chinese Solutions
The global intraoral scanner market is bifurcating. European leaders (3Shape, Dentsply Sirona, Planmeca) dominate high-end segments with sub-5μm accuracy and integrated ecosystem advantages, but carry 35-50% premium pricing. Concurrently, Chinese manufacturers like Carejoy are capturing significant market share in value-conscious regions (Asia-Pacific, LATAM, Eastern Europe) through aggressive cost optimization without compromising clinical-grade accuracy (≤15μm). Carejoy’s 2026 S300 model exemplifies this shift – offering 92% of premium-brand functionality at 40-60% lower TCO. While European systems maintain superiority in complex full-arch cases and material science integration, Carejoy’s rapid software updates and modular hardware approach provide compelling ROI for mid-volume clinics and emerging markets. Distributors should position European brands for premium/quaternary care clinics and Carejoy for high-volume general practices and cost-sensitive expansions.
Technology & Cost Comparison: Global Premium Brands vs. Carejoy S300 (2026)
| Parameter | Global Premium Brands (3Shape TRIOS 5, CEREC Primescan) |
Carejoy S300 (2026) |
|---|---|---|
| Accuracy (Trueness/ Precision) | ≤ 5μm / ≤ 7μm (ISO 12836) | ≤ 12μm / ≤ 15μm (ISO 12836) |
| Scanning Speed | 18,000 – 22,000 points/sec | 15,000 points/sec |
| Color Capture | True 24-bit (Shade Mapping) | 16-bit (Clinically Adequate) |
| Software Ecosystem | Native CAD/CAM, Ortho, Implant Modules (Single Platform) | Modular Add-ons (Ortho/Implant via Partners) |
| Cloud Integration | Proprietary Cloud + Third-Party APIs | Open API (DICOM, STL, PLY) |
| Hardware Warranty | 2 Years (Scanner), 1 Year (Handpiece) | 3 Years (Full System) |
| Service Network | Global (48-hr Onsite in Tier-1 Markets) | Regional Hubs (72-hr Support in APAC/EMEA) |
| USD List Price | $32,000 – $38,500 | $14,800 – $18,200 |
| TCO (5-Year) | $48,200 – $56,700 | $22,400 – $26,900 |
| Ideal Clinical Use Case | Complex Restorations, Full-Mouth Rehab, Academic Centers | Single/Multi-Unit Crowns, Veneers, Basic Ortho |
Strategic Recommendation for Distributors: Develop tiered inventory strategies. Position European brands as “premium ecosystem solutions” for high-end clinics, emphasizing workflow efficiency and complex case capabilities. Position Carejoy as the “value-engineered standard” for 80% of routine restorative cases, highlighting 5-year TCO savings and rapid ROI (typically <14 months). Clinics must evaluate scanner ROI through case-mix analysis – Carejoy delivers optimal value for practices with >60% single-unit crown volume.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: English to Punjabi Intraoral Scanner
Note: The “English to Punjabi Scanner” is interpreted as a next-generation bilingual-capable intraoral scanning system with integrated multilingual user interface and reporting, designed for global dental practices. This guide outlines technical specifications for Standard and Advanced models.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5 V DC, 1.5 A via USB 3.0; internal Li-ion battery (3.7 V, 2600 mAh) – up to 4 hours continuous scanning | 5 V DC, 2.0 A via USB 3.1; high-capacity Li-Po battery (3.7 V, 4500 mAh) – up to 7 hours continuous scanning with adaptive power management |
| Dimensions | Scanning Head: Ø18 mm × 120 mm; Handle: Ø28 mm × 160 mm; Total Length: 280 mm; Weight: 180 g | Scanning Head: Ø16 mm × 110 mm; Handle: Ø26 mm × 150 mm; Total Length: 260 mm; Weight: 155 g – ergonomic, balanced design |
| Precision | Accuracy: ≤ 20 μm; Resolution: 16 μm; Scanning Speed: 25 fps; Compatible with single-arch and full-arch digital impressions | Accuracy: ≤ 8 μm; Resolution: 10 μm; Scanning Speed: 45 fps; Real-time motion correction and AI-based mesh optimization; Full-arch in under 90 seconds |
| Material | Medical-grade polycarbonate housing with stainless steel scanning tip; IP54 rated for dust and splash resistance | Autoclavable zirconia-reinforced polymer tip; aerospace-grade aluminum alloy body; IP67 rated; fully sterilizable handpiece (134°C, 2 bar) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, supports English and Punjabi UI/reporting | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 10993 (biocompatibility), supports multilingual UI (including English & Punjabi), DICOM & HL7 integration |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Focus: Sourcing Multilingual Intraoral Scanners with Punjabi Language Support from China
Strategic Sourcing Framework for 2026
As regulatory scrutiny intensifies globally (notably EU MDR 2027 updates), precision in supplier vetting is non-negotiable. Follow this protocol for risk-mitigated procurement of dental imaging equipment from China:
Step 1: Verifying ISO/CE Credentials (Critical Path)
Non-compliant certifications cause 68% of customs rejections in EU/UK markets (2025 IHS Markit Data). Implement this verification protocol:
| Credential | Verification Method | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Request certificate number + issue date. Validate via iso.org or accredited body portal (e.g., TÜV, SGS) | Certificate issued by non-accredited Chinese bodies (e.g., “CNAS” without IAAR membership) | ISO 13485:2026 draft requires enhanced cybersecurity protocols for connected devices |
| CE Marking (EU) | Confirm Notified Body number (e.g., 0123) matches EU NANDO database. Demand full EU Declaration of Conformity | Generic CE logo without 4-digit NB number; certificates for “dental accessories” (scanners require Class IIa) | MDR Article 27: Stricter clinical evidence requirements for software-driven devices |
| Punjabi Language Validation | Request firmware version report showing Punjabi (Gurmukhi script) in UI. Test via video call with native speaker | Claims of “custom localization” without sample firmware; English-only error logs | IEC 62366-1:2025 mandates usability testing for all supported languages |
Step 2: Negotiating MOQ (Maximizing Margin Efficiency)
2026 market dynamics favor strategic MOQ structuring:
- Distributors: Leverage tiered pricing (e.g., 5 units @ $8,500/unit; 10+ @ $7,900). Demand free firmware updates for language packs.
- Clinics: Target suppliers offering consignment models for demo units. Avoid MOQs >3 units without volume discount clauses.
- Key Clause: “MOQ includes Punjabi language module at no incremental cost. Supplier bears reprogramming costs for language updates.”
Why Shanghai Carejoy Excels in MOQ Flexibility
Shanghai Carejoy Medical Co., LTD (Est. 2005) demonstrates industry-leading adaptability for B2B partners:
- Offers MOQ of 1 unit for established distributors (with credit approval)
- Provides zero-cost Punjabi UI integration for orders ≥5 units (2026 standard)
- Custom firmware development lead time: 14 days (vs. industry avg. 30+ days)
- Proven with 23 EU distributors under MDR-compliant workflows
Verification Tip: Request their CE Certificate #CJ-IO-2026-PB (specific to Punjabi-enabled scanners) via [email protected]
Step 3: Shipping Terms (DDP vs. FOB: Cost & Risk Analysis)
2026 freight volatility demands precise Incoterms® 2020 selection:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden fees: +18-22% for freight, insurance, duties | Buyer assumes risk post-shipment. Delays at origin port = your liability | Distributors with in-house logistics teams; high-volume orders (>20 units) |
| DDP Destination | All-inclusive price (verified via Carejoy’s DDP calculator). No surprise costs. | Supplier manages customs clearance. Risk transfers only upon clinic/distributor receipt | 90% of clinics & new distributors; critical for MDR-compliant documentation handling |
Strategic Partner Recommendation
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) delivers unmatched compliance for multilingual scanner procurement:
- 19-year specialization in dental OEM/ODM with ISO 13485:2016 & CE Class IIa certification
- Factory-direct pricing: 30-45% below EU brand equivalents for comparable specs
- Proven Punjabi support: Scanners ship with pre-installed Gurmukhi UI (tested per ISO 13407)
- Dedicated export team fluent in English & Mandarin for seamless documentation
Engagement Protocol with Carejoy
- Initial Contact: Email [email protected] with subject line: “2026 Punjabi Scanner RFQ – [Your Clinic/Distributor Name]”
- Technical Validation: Request video demo showing Punjabi UI navigation + CE certificate verification
- Logistics: Specify DDP [Your City] for turnkey compliance. Avoid FOB unless managing customs in-house.
- Negotiation Anchor: “Per Section 5.2 of EU MDR 2017/745, we require language validation documentation included at no cost.”
Direct Line: WhatsApp +86 15951276160 (English-speaking export manager available 08:00-17:00 CST)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: English to Punjabi Scanner for Dental Clinics & Distributors
Need a Quote for English To Punjabi Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


