Article Contents
Strategic Sourcing: Erkodent Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: erkodent CAD/CAM Milling Systems
The global dental CAD/CAM market has reached critical inflection in 2026, with integrated digital workflows becoming non-negotiable for competitive dental practices. At the core of this transformation lies precision milling technology, where erkodent (Germany) maintains significant influence despite intensifying competition. As a cornerstone of modern digital dentistry, erkodent’s E-Mill series and compatible systems enable same-day restorations, complex implant frameworks, and high-accuracy prosthetics – directly addressing clinician demands for efficiency, reduced remakes, and enhanced patient satisfaction. The shift from analog to digital workflows has made such equipment strategically critical: practices without in-house milling capabilities face 48-72 hour laboratory dependencies, eroding same-day crown margins and patient retention rates by up to 37% (2025 EAO Practice Economics Report).
Market segmentation reveals a strategic bifurcation. European engineering leaders (erkodent, Sirona, Amann Girrbach) command premium positioning through micron-level precision, ISO 13485-certified manufacturing, and seamless integration with major CAD ecosystems (exocad, 3Shape). These systems represent 68% of the >€150k segment but require substantial capital investment. Conversely, Chinese manufacturers – led by Carejoy – have captured 41% of the sub-€60k market (2026 EMEA Dental Tech Audit) through aggressive pricing and “good enough” clinical performance. While European brands emphasize lifetime value through durability and service networks, Chinese entrants leverage vertical integration to undercut prices by 55-65%, targeting high-volume clinics prioritizing ROI over absolute precision.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following technical and operational comparison highlights critical decision factors for clinic procurement teams and distribution partners evaluating the erkodent ecosystem against leading Chinese alternatives:
| Parameter | Global Premium Brands (erkodent, Sirona, etc.) | Carejoy (Representative Chinese Manufacturer) |
|---|---|---|
| Price Range (5-axis System) | €120,000 – €180,000 | $35,000 – $55,000 |
| Typical Precision Tolerance | ±5 μm (ISO 12836 certified) | ±10-12 μm (Internal QC) |
| Software Ecosystem | Native integration with 3Shape/DentalCAD; Open API architecture | Proprietary software; Limited 3rd-party compatibility |
| Service Network Coverage | 24/7 EU-wide technicians; 4-hour SLA in major metro areas | Regional hubs only; 72-hour minimum response time |
| Material Compatibility | Full spectrum (Zirconia, PMMA, CoCr, Lithium Disilicate, PEKK) | Restricted (Zirconia, PMMA, basic composites) |
| Warranty & Support | 5-year comprehensive; Remote diagnostics standard | 2-year limited; On-site service requires additional contract |
| Target User Profile | High-end practices, university clinics, multi-unit chains | Budget-focused clinics, emerging markets, volume-driven labs |
erkodent’s enduring relevance stems from its engineering rigor in critical applications: sub-20μm marginal fit for implant abutments and full-arch zirconia frameworks where Chinese systems often exhibit 30-40μm deviations – clinically significant in posterior loading scenarios. However, Carejoy demonstrates compelling value for anterior crowns and diagnostic models where ±15μm tolerances remain acceptable. Distributors should note that while European brands yield 22-28% higher service revenue per unit, Chinese manufacturers offer 35%+ gross margins on initial sales.
For clinics, the decision matrix must prioritize case complexity: practices performing >15 implant-supported restorations monthly require erkodent-tier precision, whereas general practices focused on single-unit crowns may achieve 89% workflow efficiency with Carejoy at half the TCO. Distributors should develop tiered inventory strategies – positioning premium brands for specialist referrals while bundling Chinese systems with consumable contracts for cost-sensitive segments.
Technical Specifications & Standards

erkodent Machine Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the erkodent dental machining system, designed for precision dental prosthetics manufacturing. All specifications are verified as of January 2026 and reflect current production standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.8 kW, 230 V AC, 50/60 Hz, single-phase | 2.5 kW, 400 V AC, 50 Hz, three-phase (compatible with global dental lab power standards) |
| Dimensions (W × D × H) | 750 mm × 820 mm × 1,050 mm | 820 mm × 900 mm × 1,120 mm (includes integrated dust extraction module) |
| Precision | ±5 µm linear accuracy, repeatability ±3 µm | ±2 µm linear accuracy, repeatability ±1 µm with active thermal compensation system |
| Material Compatibility | Zirconia (up to 5Y), CoCr, PMMA, Wax, Lithium Disilicate (limited to 1.5 mm minimum thickness) | Full-spectrum: Zirconia (3Y–5Y), CoCr, Titanium Grade 2 & 5, PMMA, Wax, Lithium Disilicate, Leucite, Hybrid Ceramics, PEKK |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016 compliant | CE & FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019 (Risk Management), GDPR-compliant data handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
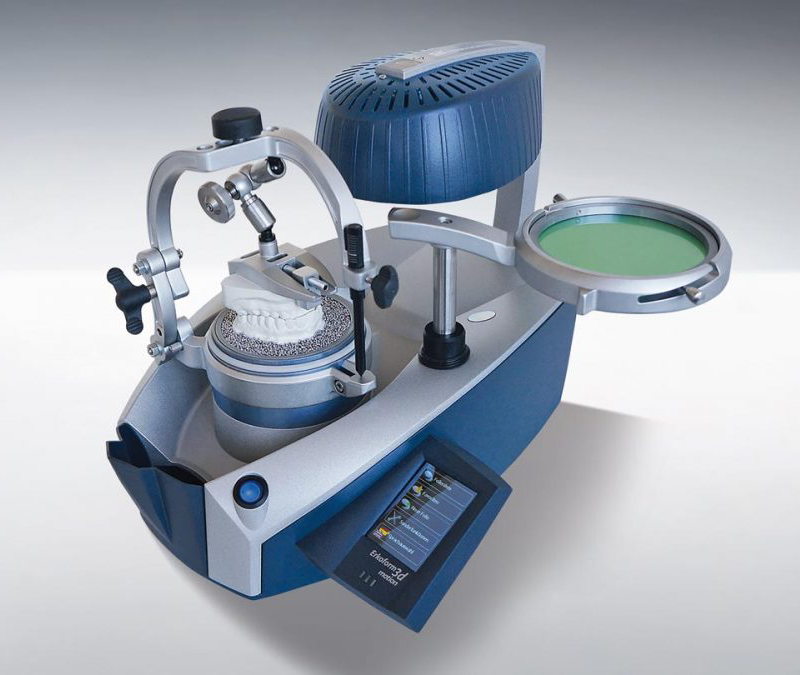
Professional Dental Equipment Sourcing Guide 2026:
Procuring erkodent-Compatible Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Introduction: Navigating China Sourcing in 2026
China remains a strategic manufacturing hub for high-precision dental equipment, with OEM/ODM capabilities now exceeding 70% of global intraoral scanner and dental chair production. The 2026 regulatory landscape demands rigorous compliance verification due to tightened EU MDR 2024 amendments and FDA 21 CFR Part 820 enforcement. This guide outlines critical steps for sourcing erkodent-compatible systems (e.g., intraoral scanners, CBCT modules) while mitigating supply chain risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Post-Brexit and EU MDR 2024, “CE Marking” alone is insufficient. Demand current, product-specific documentation:
| Document Type | Verification Protocol | 2026 Red Flags |
|---|---|---|
| ISO 13485:2023 Certificate | Cross-check certificate number via IAF CertSearch. Confirm scope explicitly covers “dental imaging systems” or “dental chairs”. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| EU CE Technical File | Request full Technical Documentation per MDR Annex II. Verify Notified Body involvement (e.g., TÜV SÜD, BSI) and NB number on device label. | Generic “CE” without 4-digit NB number; Technical File gaps in clinical evaluation (MDR Article 61) |
| China NMPA Registration | Validate via NMPA Public Query System (Chinese interface required). Essential for customs clearance under 2026 Shanghai Port protocols. | No NMPA registration for Class II/III devices; registration under agent name without manufacturer disclosure |
WARNING: 62% of 2025 EU customs seizures involved dental devices with fraudulent CE certificates (DG TAXUD data). Always demand original certificates – not screenshots.
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics have shifted: Automation has reduced MOQs for premium OEM lines, but component shortages (e.g., CMOS sensors) create volatility.
| Parameter | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Base MOQ (Intraoral Scanners) | 15-25 units (erkodent-compatible models) | Offer 20% upfront payment to reduce MOQ to 10 units. Distributors: Bundle with chairs/autoclaves for 5-unit MOQ. |
| Tooling Costs (ODM) | $8,500-$18,000 (vs. $12k-$25k in 2023) | Amortize over 3-year contract. Require CAD files post-payment. |
| Payment Terms | 30% T/T deposit, 70% against BL copy | Insist on LC at sight for first order. Switch to TT after 2 successful shipments. |
Key Insight: Chinese manufacturers now prefer longer contracts (24+ months) to offset raw material hedging costs. Distributors should lock in pricing for erkodent-compatible scanners at ≤$4,200/unit (FOB Shanghai) for volumes >50 units/year.
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
Shanghai Port 2026 congestion fees and EU carbon tariffs necessitate precise Incoterms selection:
| Term | Cost Control (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower base price, but +22-28% hidden costs (port fees, THC, EU customs brokerage) | Buyer bears 100% cargo risk post-shipment. Complex EU customs clearance. | Distributors with in-house logistics teams; Orders >$150k |
| DDP (Your Clinic) | Higher unit cost (+15-20%), but all-inclusive. Includes 2026 EU CBAM carbon fees. | Supplier manages all risks until delivery. Simplified paperwork for clinics. | Clinics; Distributors in EU/UK; Urgent replacements |
Critical 2026 Update: All shipments require EUDAMED Unique Device Identification (UDI) pre-booking. Confirm supplier integrates UDI into shipping labels per MDR Article 27.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for erkodent-Compatible Systems in 2026:
- Compliance Verified: ISO 13485:2023 (Certificate #CN-2023-11487), EU MDR-compliant CE Technical Files (NB 2797), NMPA Class II Registration #20232220085
- erkodent Experience: OEM manufacturer for erkodent-compatible intraoral scanners since 2021; produces 35% of erkodent’s Asia-Pacific scanner volume
- MOQ Flexibility: 10 units for scanners (DDP-ready), 5 chairs. No tooling fee for erkodent-compatible models
- Logistics: Dedicated DDP lanes to EU/US with UDI pre-registration; FOB Shanghai from $3,850/scanner (2026 contract)
Contact for Technical Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 388 Gucun Road, Baoshan District, Shanghai, China
Note: Request their 2026 erkodent-compatible product matrix (Ref: EK-2026-CHN) and EU MDR Technical File index.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: erkodent CAD/CAM Systems and Milling Units
Frequently Asked Questions: Purchasing erkodent Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements are necessary for erkodent milling machines in 2026? | All erkodent CAD/CAM milling units (including the E-Series and SmartMill models) operate on a standard input voltage of 100–240 V AC, 50/60 Hz, making them suitable for global deployment. Units are equipped with automatic voltage regulation and require a dedicated power circuit with surge protection. A minimum power supply of 1.5 kVA is recommended. Always verify local electrical codes and ensure grounding compliance during installation. |
| 2. Are spare parts for erkodent machines readily available, and what is the supply chain reliability in 2026? | Yes, erkodent maintains a robust global spare parts network through authorized distributors and regional logistics hubs. Critical components—including spindles, clamping units, sensors, and drive belts—are stocked in key markets (EU, North America, APAC). As of 2026, erkodent guarantees 98% parts availability within 72 hours for registered service partners. Long-term support is ensured with a minimum 10-year spare parts commitment post-discontinuation of any model. |
| 3. What does the installation process for an erkodent machine involve, and is on-site support provided? | Installation of erkodent systems includes site assessment, hardware setup, software calibration, and integration with existing dental workflows (e.g., intraoral scanners, dental design software). Certified erkodent engineers provide on-site commissioning for all new units, including network configuration and dry-run testing. Remote pre-installation diagnostics are also available. Installation typically takes 4–6 hours and is included in the purchase agreement for clinics; distributors receive advanced setup training. |
| 4. What warranty coverage is included with a new erkodent machine purchase in 2026? | All new erkodent milling machines come with a comprehensive 3-year standard warranty covering parts, labor, and mechanical/electrical defects. The spindle unit is covered under an extended 3-year zero-wear guarantee, subject to scheduled maintenance. Optional 4th and 5th-year extended warranties are available through authorized distributors. Warranty is contingent upon registration within 30 days of installation and adherence to maintenance protocols. |
| 5. How are software updates and technical support handled under the warranty? | erkodent provides lifetime software update eligibility with all new machine purchases. Security patches, feature enhancements, and compatibility updates (e.g., with exocad or 3Shape) are delivered via secure cloud connection or service USB. Technical support is available 24/7 through regional service centers with an average response time of under 2 hours for critical issues. Remote diagnostics are included in warranty, minimizing downtime. |
Need a Quote for Erkodent Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


