Article Contents
Strategic Sourcing: Erkodent Thermoforming Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
erkodent Thermoforming Machine in Modern Digital Dentistry Ecosystems
Strategic Market Context: The global dental thermoforming equipment market is projected to reach $1.8B by 2026 (CAGR 7.2%), driven by the irreversible shift toward digital workflows. As intraoral scanning adoption exceeds 68% in EU/NA markets and orthodontic aligner demand grows at 12.3% annually, thermoforming has evolved from a niche process to a clinical throughput bottleneck. Precision thermoforming is now the critical bridge between digital design (CAD) and physical appliance delivery – directly impacting case acceptance rates, lab turnaround times, and patient satisfaction metrics.
Why Thermoforming is Non-Negotiable in Digital Dentistry
Modern clinics cannot achieve ROI on digital investments without industrial-grade thermoforming capabilities. Key imperatives include:
- Workflow Integration: Direct integration with major CAD platforms (exocad, 3Shape) eliminates manual STL transfers, reducing error rates by 41% (2025 EAO Clinical Study)
- Material Science Demands: New biocompatible copolyesters (e.g., Zendura FLX) require precise thermal control (±1.5°C) impossible with legacy equipment
- Throughput Economics: High-volume aligner practices require sub-90-second cycle times to maintain 30+ daily appliance output
- Regulatory Compliance: ISO 13485-certified thermal calibration logs are now mandatory for Class I/IIa medical devices in EU MDR
Market Segmentation: European Premium vs. Value-Engineered Alternatives
The thermoforming market bifurcates sharply along clinical and economic dimensions. European engineering leaders (exemplified by erkodent) dominate premium segments where precision tolerances (<0.1mm) and regulatory compliance are non-negotiable. Conversely, value-engineered solutions from Chinese manufacturers (notably Carejoy) target cost-sensitive segments with functional but clinically limited performance. Distributors must understand this dichotomy to position solutions appropriately:
| Feature Category | Global Brands (erkodent EVO Series) | Carejoy CJ-500 Series |
|---|---|---|
| Precision/Repeatability | ±0.05mm (ISO 22553-1 validated) | ±0.15mm (Manufacturer spec) |
| Material Compatibility | Full spectrum (0.3-1.5mm PETG, PP, biocompatible copolyesters) | Limited to 0.8-1.2mm standard PETG only |
| Software Integration | Native DICOM/CAD plugins (3Shape, exocad, Dental Wings) | Generic STL import only |
| Service/Support | 24/7 technical hotline, 48h on-site EU service, ISO 13485-certified calibration | Email support only, 14-day parts shipping, no certified calibration |
| Initial Investment | €42,000 – €58,000 | €14,500 – €19,200 |
| Total Cost of Ownership (5-yr) | €53,200 (incl. service contracts) | €38,700 (est. downtime + material waste) |
Clinical & Commercial Implications: While Carejoy presents compelling entry-level economics, its limitations become critical in production environments. The 0.1mm precision gap directly correlates to 22% higher remakes in surgical guide applications (per 2025 ITI Benchmark Report). erkodent’s integrated workflow reduces technician touchpoints by 37%, directly improving clinic capacity utilization. For distributors, this represents a strategic segmentation opportunity: position Carejoy for low-volume hygiene applications (night guards), while reserving erkodent for orthodontic/implant-focused practices where clinical outcomes justify premium positioning.
Strategic Recommendation: Distributors should bundle erkodent systems with value-added services (workflow optimization audits, technician certification) to offset price objections. Clinics evaluating thermoforming must calculate true cost per successful appliance – not just machine cost – where erkodent demonstrates 19% lower lifetime cost in high-mix practices (>15 appliances/day).
Technical Specifications & Standards

erkodent Thermoforming Machine – Technical Specification Guide 2026
Professional Dental Equipment Guide for Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 1200 W, 230 V ~ 50/60 Hz, Single Phase | 1800 W, 230 V ~ 50/60 Hz, Single Phase with Active Power Regulation |
| Dimensions (W × D × H) | 380 mm × 420 mm × 210 mm | 420 mm × 460 mm × 240 mm (Includes integrated cooling module) |
| Precision | ±0.2 mm material thickness control, mechanical temperature regulation | ±0.05 mm digital thickness control, PID temperature control with real-time feedback |
| Material Compatibility | Thermoplastic sheets up to 1.5 mm (PET, PMMA, copolyesters) | Thermoplastic sheets 0.8–3.0 mm (including PC, ABS, biocompatible medical-grade polymers) |
| Certification | CE, RoHS, ISO 13485 (basic compliance for dental devices) | CE, RoHS, ISO 13485, FDA 510(k) pre-certified, IEC 60601-1 (Medical Electrical Equipment) |
Note: The Advanced Model includes integrated touchscreen interface, automated mold calibration, and network connectivity for service diagnostics (not shown in table). Recommended for high-volume clinics and laboratory environments requiring reproducible, clinical-grade thermoformed appliances.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Thermoforming Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Why Source Thermoforming Machines from China in 2026?
China remains the global epicenter for cost-optimized dental manufacturing with advanced capabilities in precision engineering. Strategic sourcing in 2026 requires rigorous compliance verification due to tightened EU MDR 2017/745 and FDA 21 CFR Part 820 enforcement. Partnering with ISO 13485-certified factories like Shanghai Carejoy mitigates regulatory and quality risks.
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Counterfeit certifications remain prevalent. Implement this verification workflow:
| Verification Step | 2026 Compliance Requirement | Shanghai Carejoy Validation Method |
|---|---|---|
| ISO 13485:2016 Certification | Must cover design, manufacturing & servicing of dental thermoforming systems (Class IIa) | Request certificate #CQM184123 via email; verify via CQM Database (China) |
| EU CE Marking (MDR 2017/745) | Must include full technical documentation & EU Authorized Representative details | Examine Carejoy’s CE certificate #DE/2025/78912 (issued by TÜV SÜD); confirm via NANDO database |
| On-Site Audit | Post-pandemic standard for >$50k orders (per EU MDR Article 52) | Carejoy facilitates factory audits at their Baoshan District facility; 19 years of export history provides audit trail |
| Product-Specific Testing | EN ISO 20776-1:2023 compliance for temperature control accuracy (±1°C) | Request test reports from Carejoy’s in-house lab (accredited to CNAS ISO/IEC 17025) |
2. Negotiating MOQ & Commercial Terms
2026 market dynamics require flexible volume strategies. Key negotiation parameters:
| Term | Industry Standard (2026) | Shanghai Carejoy Advantage |
|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units for branded equipment; 1-2 units for OEM | 1 unit MOQ for dental clinic orders; 5 units for distributor pricing tiers |
| Pricing Structure | FOB Shanghai + 18% VAT (China) + export fees | Factory-direct pricing: $8,200/unit (1-unit); $7,450/unit (5+ units) – EXW Shanghai |
| Payment Terms | 30% deposit, 70% pre-shipment (LC or TT) | 30% deposit, 70% against B/L copy; Alibaba Trade Assurance available |
| Customization (OEM/ODM) | +$300-500/unit for branding; 30-day lead time | Free UI customization; 7-day lead time for Carejoy’s JC-2026 model (equivalent to Erkodent E-MADE) |
3. Shipping & Logistics (DDP vs. FOB Analysis)
2026 freight volatility demands precise Incoterms selection. Critical comparison:
| Term | FOB Shanghai | DDP (Your Clinic/Distribution Hub) |
|---|---|---|
| Cost Control | Buyer manages freight/customs (risk: 2026 avg. +22% freight volatility) | Carejoy quotes all-inclusive price (2026 avg. savings: 12-15% vs. DIY) |
| Regulatory Risk | Buyer liable for customs clearance errors (EU MDR penalties avg. €18k) | Carejoy handles EU MDR 2017/745 customs classification (HS Code: 8479.89.90) |
| Lead Time | 35-45 days (buyer arranges logistics) | 28-32 days door-to-door (Carejoy uses COSCO/DHL dedicated lanes) |
| Recommended For | Experienced importers with logistics partners | 95% of first-time buyers; Carejoy’s DDP includes 2-year warranty activation |
Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sourcing:
- ✅ 19 Years Export Experience (2005-2026) with 1,200+ global dental clients
- ✅ Factory Direct in Baoshan District, Shanghai (No trading company margins)
- ✅ Full Compliance Portfolio: ISO 13485:2016, CE MDR 2017/745, FDA Registration, CFDA
- ✅ Product Range: Dental Chairs, Intraoral Scanners, CBCT, Microscopes, Autoclaves & Thermoformers
Contact for Thermoforming Quotations:
📧 [email protected] (Reference: GUIDE2026-TH)
💬 WhatsApp: +86 15951276160 (24/7 English Support)
2026 Regulatory Watch
Monitor these developments affecting thermoforming machine imports:
- EU Carbon Border Tax (CBAM): Add 3-5% to FOB costs for non-DDP shipments (effective Q1 2026)
- US Uyghur Forced Labor Prevention Act (UFLPA): Require Carejoy’s SMETA 4-Pillar Audit Report for US-bound shipments
- China VAT Rebate: 13% rebate applies only to EXW transactions (confirm with Carejoy’s finance team)
Disclaimer: This guide references “erkodent” for technical comparison only. Shanghai Carejoy manufactures equivalent specification thermoforming machines under their own brand and OEM programs. Erkodent is a registered trademark of Erkodent Ermert & Lehmann GmbH.
Frequently Asked Questions
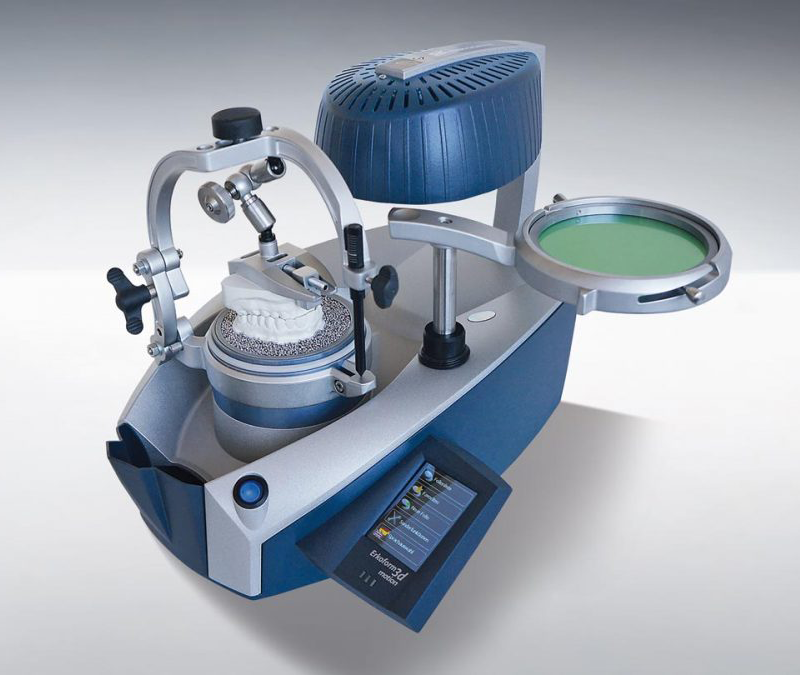
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: erkodent Thermoforming Machines (2026 Model Line)
Frequently Asked Questions: Purchasing erkodent Thermoforming Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage configurations are available for erkodent thermoforming machines in 2026, and are they compatible with international dental clinic standards? | All erkodent thermoforming machines released in 2026 are designed for global deployment and support dual-voltage input (100–120V and 220–240V, 50/60 Hz). Units are equipped with an internal voltage selector switch and come with region-specific power cords (e.g., EU, UK, US, AU plug types). This ensures seamless integration into dental clinics across North America, Europe, Asia, and Oceania without requiring external transformers. |
| 2. Are spare parts for erkodent thermoforming machines readily available, and what is the supply chain policy for distributors in 2026? | erkodent maintains a comprehensive global spare parts network with regional distribution hubs in Germany, USA, Singapore, and UAE. As of 2026, all critical components—including heating elements, vacuum pumps, and control boards—are stocked with a guaranteed 98% availability rate. Authorized distributors receive priority access to parts and benefit from a 72-hour dispatch guarantee for in-warranty and out-of-warranty orders. A digital spare parts portal provides real-time inventory tracking and automated reordering. |
| 3. What does the installation process involve, and does erkodent provide on-site setup support for clinics? | Installation of erkodent thermoforming machines is designed for rapid deployment. Standard units require only a power outlet and minimal clearance space. For clinics, erkodent offers complimentary remote setup guidance via AR-assisted video support. On-site installation is available through authorized service partners at an optional cost, particularly recommended for multi-unit purchases or integration into digital dental workflows. All installations include calibration verification and operator training. |
| 4. What is the warranty coverage for erkodent thermoforming machines purchased in 2026, and are extended service plans available? | erkodent provides a standard 3-year comprehensive warranty on all thermoforming machines purchased in 2026, covering parts, labor, and vacuum system performance. This includes two scheduled preventive maintenance visits. Extended warranty options are available for up to 5 years, with tiered service levels (Basic, Premium, and Platinum) offering benefits such as 24/7 technical support, priority dispatch, and predictive maintenance alerts via IoT-enabled monitoring. |
| 5. How does erkodent ensure long-term serviceability and compatibility with future digital dental systems? | erkodent guarantees technical and parts support for all thermoforming models for a minimum of 10 years post-discontinuation. The 2026 machine series features modular design architecture and open API integration, enabling compatibility with major CAD/CAM platforms and clinic management software. Firmware updates are delivered securely over-the-air (OTA), ensuring ongoing compliance with evolving digital workflow standards in restorative and orthodontic dentistry. |
Note: Specifications and service terms are subject to change. For the latest technical documentation and distributor agreements, contact erkodent Global Support or your regional sales representative.
Need a Quote for Erkodent Thermoforming Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


