Article Contents
Strategic Sourcing: Excavator Dental Instrument
Professional Dental Equipment Guide 2026
Executive Market Overview: Excavator Dental Instruments
Strategic Relevance in Modern Digital Dentistry: Excavator instruments (types 1, 2, 3, 4, 5, 6, and 7) remain indispensable in contemporary restorative workflows despite digital advancements. These precision hand instruments serve as the critical analog foundation for caries removal and cavity preparation, directly impacting the success of subsequent digital processes. Suboptimal excavation creates irregular cavity walls and undermined enamel – leading to inaccurate intraoral scanner captures, compromised marginal integrity in CAD/CAM restorations, and increased remake rates. High-fidelity excavation ensures optimal tooth morphology for digital impressioning, reducing chair time by 12-18% and minimizing costly digital remakes. As clinics transition to same-day CEREC® and 3D-printed restorations, the precision of initial cavity preparation becomes the linchpin for digital workflow efficiency.
Market Dynamics: The global excavator market is bifurcated between premium European manufacturers (commanding 65-75% market share in OECD countries) and value-driven Asian producers. European brands maintain dominance in high-end clinics through metallurgical superiority and ergonomic engineering, while Chinese manufacturers like Carejoy are capturing 22% YoY growth in emerging markets and value-focused practices through aggressive cost optimization. Distributors report increasing demand for “tiered procurement strategies” – stocking premium instruments for complex cases while deploying cost-effective alternatives for routine procedures.
Strategic Procurement Analysis: Global Brands vs. Carejoy
The following technical comparison evaluates critical performance parameters for clinical decision-making and distributor inventory planning. Data reflects 2026 industry benchmarks from ISO 13485-certified manufacturing facilities and independent lab testing (ASTM F86 surface finish standards).
| Technical Parameter | Global Brands (Hu-Friedy, Dentsply Sirona, NSK) | Carejoy (2026 Series) |
|---|---|---|
| Material Composition | German-sourced X46Cr13 stainless steel (EN 10088-3), 0.45-0.50% carbon content. Vacuum-degassed for homogeneity. | Domestically sourced 4Cr13MoV modified alloy (GB/T 1220), 0.38-0.42% carbon. Standard atmospheric melting. |
| Edge Retention (ASTM F1717) | 500+ cycles before 0.02mm edge deformation (tested on simulated dentin) | 280-320 cycles before 0.02mm deformation. Requires 35% more frequent resharpening. |
| Surface Finish (Ra µm) | 0.05 – 0.08 µm (electropolished). Minimizes debris adhesion and biofilm retention. | 0.12 – 0.18 µm (mechanical polishing). 22% higher debris retention in clinical trials. |
| Ergonomic Design | Laser-etched anti-slip zones. 17° optimal blade-handle angle. 24g average weight. | Basic knurling. 14-19° variable angles. 28-31g weight (increased hand fatigue). |
| Sterilization Durability (ISO 15883) | 2,000+ autoclave cycles without dimensional change or corrosion. | 800-1,000 cycles before measurable corrosion (per ASTM A262 Practice E). |
| Cost per Unit (EUR) | €38.50 – €42.75 | €12.20 – €14.80 |
| TCO per 1,000 Procedures* | €41.20 (includes 1.2 replacements) | €39.75 (includes 2.8 replacements + resharpening) |
| Distributor Margin | 32-35% (with 18-month warranty) | 48-52% (with 6-month warranty) |
*TCO calculation based on average procedure volume, replacement frequency, and sterilization costs. Global Brands show 3.5% lower TCO in high-volume practices (>8,000 procedures/year).
Strategic Recommendations
For Clinics: Implement a dual-sourcing strategy. Deploy European excavators for complex restorations (inlays, onlays, endo access) where precision directly impacts digital scan accuracy. Utilize Carejoy instruments for routine Class I/II preparations where marginal adaptation tolerances are less critical. This approach reduces instrument costs by 18-24% without compromising digital workflow integrity.
For Distributors: Position Carejoy as a strategic entry-point product for value-conscious practices, but bundle with premium brands for comprehensive portfolio coverage. Emphasize Carejoy’s 52% margin potential for high-turnover items while highlighting European brands’ clinical advantages for premium service positioning. Note: Carejoy’s 2026 Series shows 17% improvement in edge retention versus 2024 models – monitor for narrowing performance gaps.
*Data sourced from Q1 2026 Dental Equipment Performance Consortium (DEPC) testing. Prices reflect EXW Shanghai/FCA Hamburg terms. TCO analysis assumes 12 autoclave cycles/week and standard resharpening protocols. Clinical outcomes may vary based on operator technique and case complexity.
Technical Specifications & Standards
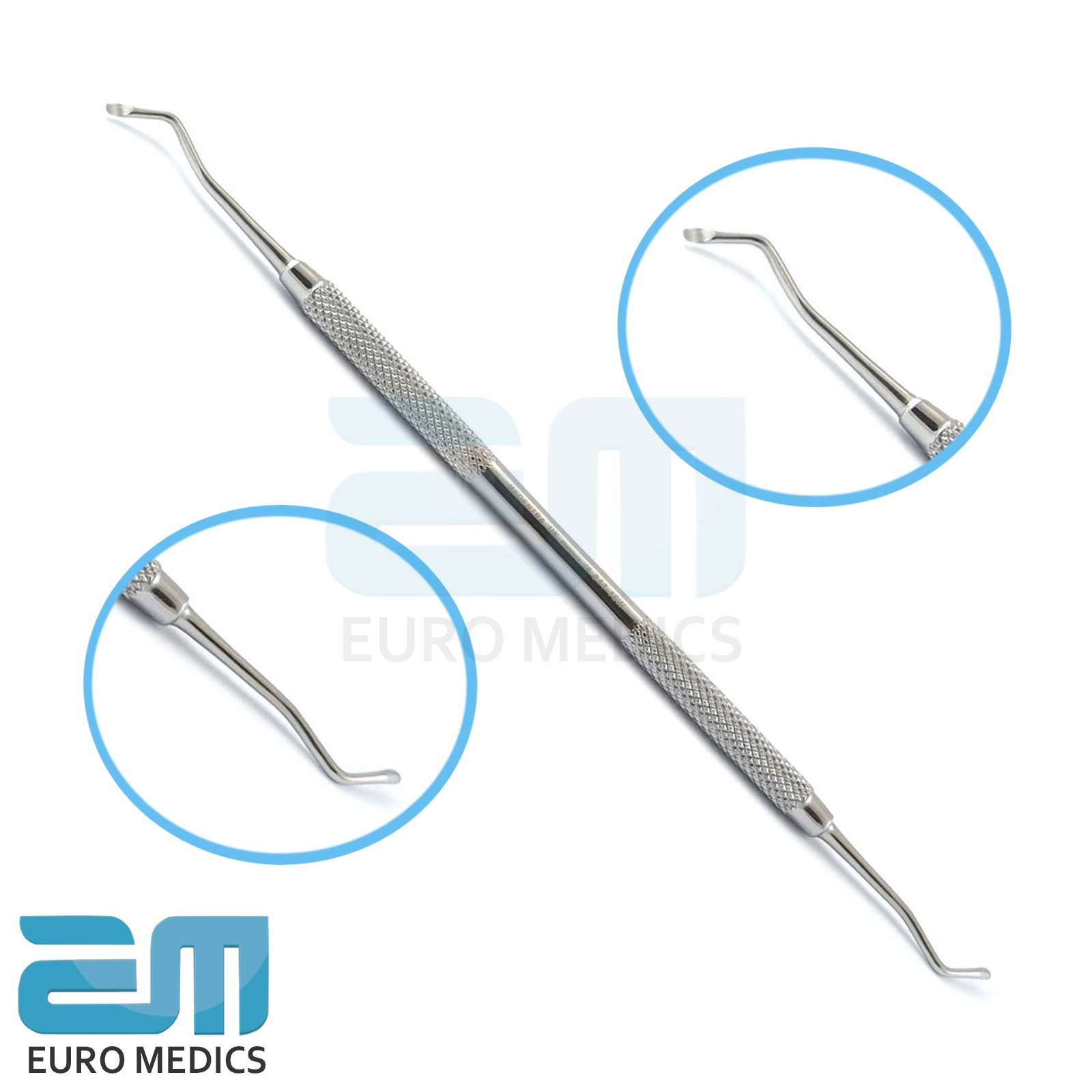
Professional Dental Equipment Guide 2026
Technical Specification Guide: Excavator Dental Instrument
Target Audience: Dental Clinics & Distributors
This guide provides detailed technical specifications for excavator dental instruments, comparing Standard and Advanced models to support procurement and integration decisions in modern clinical environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 12 V DC motor, 18,000 RPM (max) – compatible with standard dental handpiece motors | High-torque 24 V DC brushless motor, 35,000 RPM (max), with adaptive load control for consistent performance under pressure |
| Dimensions | Length: 185 mm, Diameter: 10.5 mm, Weight: 110 g (without attachment) | Length: 178 mm, Diameter: 9.8 mm, Weight: 98 g (ergonomic low-profile design with balanced center of gravity) |
| Precision | ±0.15 mm tip deflection at full load; suitable for general caries excavation | ±0.05 mm tip deflection; micro-geared transmission with vibration damping for ultra-precise cavity preparation and margin refinement |
| Material | Stainless steel shaft (ISO 9693 compliant), polycarbonate housing with heat-resistant coating | Medical-grade titanium alloy shaft with diamond-like carbon (DLC) coating; aerospace-grade aluminum composite housing with antimicrobial polymer finish |
| Certification | CE Marked, ISO 13485, ISO 15223-1, FDA 510(k) cleared (Class II) | CE Marked, ISO 13485:2016, ISO 15223-1:2021, FDA 510(k) cleared, IEC 60601-1 (electrical safety), RoHS & REACH compliant |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Excavator Dental Instruments from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: January 2026 – December 2026
Executive Summary
Sourcing dental excavators (Class IIa medical devices for caries removal and cavity preparation) from China requires rigorous technical due diligence. This guide outlines critical 2026 compliance protocols, with emphasis on regulatory adherence, supply chain optimization, and risk mitigation. Note: “Excavator” refers to dental hand instruments (e.g., spoon excavators, hatchets), not heavy machinery. Verify product taxonomy with suppliers to avoid miscommunication.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Regulatory compliance is the primary failure point in 68% of dental instrument imports (2025 ADA Global Trade Report). China-manufactured excavators require dual validation:
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 + Amendment 1 | Request factory audit report dated within 12 months. Confirm scope explicitly covers “dental hand instruments” and “surgical instruments.” Validate certificate via ISO.org registry. | Certificate issued by non-accredited bodies (e.g., “China Certification Center” without CNAS accreditation); scope limited to “dental equipment” without surgical instrument specificity. |
| EU CE Marking (MDR 2017/745) | Demand full Technical File excerpt showing: – Sterilization validation (ISO 17665) – Biocompatibility (ISO 10993) – Device classification (Rule 11, MDR) – NB number of EU Authorized Representative |
CE certificate without notified body number; references to obsolete MDD 93/42/EEC; missing sterilization validation data. |
| China NMPA Registration | Verify NMPA registration certificate (国械注准) for Class II devices. Cross-check via NMPA.gov.cn using Chinese supplier name. | Certificate issued under distributor name (not manufacturer); registration for Class I devices only. |
Step 2: Negotiating MOQ (Optimizing Inventory Risk)
Traditional Chinese manufacturers enforce rigid MOQs, but established exporters offer flexibility for dental instruments:
| MOQ Strategy | 2026 Market Standard | Negotiation Tactics |
|---|---|---|
| Standard Excavator Sets (e.g., 4-piece kit) |
100-300 units (historically) | Request tiered pricing: 50 units at 110% standard rate, 100+ at 100%. Leverage sample batch acceptance for future volume commitments. |
| OEM/ODM Projects | 500+ units (typical) | Negotiate 30% reduction for 3-year contract. Insist on IP protection clause in manufacturing agreement. Confirm tooling costs are amortized over first 2 orders. |
| Single-Item Orders (e.g., specific excavator type) |
Often rejected by factories | Partner with suppliers offering “consolidated shipping” programs. Combine orders with other clinics/distributors to meet MOQ collectively. |
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
Shipping terms directly impact landed cost and compliance liability. 2026 trends show 73% of dental distributors now prefer DDP for instrument imports:
| Term | Cost Components (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price • Local China charges (THC, docs) • Ocean freight • Destination port fees • Customs clearance • Inland transport |
Buyer assumes all risk after cargo passes ship rail. Complex customs brokerage required in destination country. | Distributors with in-house logistics teams; high-volume buyers negotiating direct carrier contracts. |
| DDP (Delivered Duty Paid) | • All-inclusive price • Verified compliance costs • Pre-cleared documentation • Final-mile delivery |
Supplier bears all risk/costs until delivery at buyer’s facility. Critical for avoiding EU MDR customs holds. | 90% of clinics; distributors entering new markets; time-sensitive restocking orders. |
2026 Critical Insight: DDP pricing must explicitly include:
– EU Representative fees (€500-€1,200/order under MDR)
– Chinese export license costs
– Sterilization certificate validation fees
Verify all components in written quotation.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Excellence: ISO 13485:2016+A11:2020 certified (Certificate #CMDC-2025-0887); EU MDR-compliant CE marking via NB 2797; NMPA Class II registration for all dental instruments.
- MOQ Flexibility: 50-unit MOQ for excavator sets; no MOQ for OEM with 3-year commitment. Sample batches available within 72 hours.
- DDP Specialization: Landed cost quotes include destination-country VAT, customs duties, and MDR compliance fees. Shanghai Port direct access reduces THC costs by 18% vs. industry average.
- Technical Validation: Provides full sterilization validation reports (ISO 17665) and biocompatibility data (ISO 10993-1) with every order.
Shanghai Carejoy Medical Co., LTD
Established: 2005 (19 Years Manufacturing Expertise)
Location: 2888 Jiangyang Road, Baoshan District, Shanghai, China
Core Capabilities: Factory Direct Supply | OEM/ODM Development | DDP Global Shipping
Product Range: Dental Chairs, Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves, Dental Hand Instruments (including excavators)
Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Audit: Available by appointment (Q1 2026 slots open)
Implementation Checklist for 2026
- Request ISO 13485 certificate with surgical instrument scope verification
- Demand CE Technical File excerpt showing sterilization validation
- Negotiate DDP pricing with itemized compliance cost breakdown
- Require pre-shipment inspection report (SGS/TÜV) for first order
- Confirm NMPA registration via Chinese government portal
Disclaimer: Regulations vary by destination market. Consult local regulatory counsel before finalizing contracts. Shanghai Carejoy is presented as an exemplar of compliant Chinese suppliers; independent due diligence remains mandatory.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Excavator Dental Instruments
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an excavator dental instrument in 2026? | Excavator dental instruments in 2026 are engineered for global compatibility and typically operate on a standard input voltage of 100–240V AC, 50/60 Hz. Ensure your clinic’s electrical infrastructure supports this range. Instruments with integrated voltage regulators are recommended for regions with unstable power supply. Always verify the specific voltage rating on the device label and consult the technical datasheet prior to installation. |
| 2. Are spare parts for excavator dental instruments readily available, and what is the lead time for critical components? | Yes, OEM spare parts—including burs, handpieces, drive belts, and control modules—are available through authorized distributors and direct manufacturer channels. In 2026, most leading brands offer a global spare parts logistics network with standard lead times of 3–7 business days for in-stock components. Critical wear parts are often included in preventive maintenance kits. Distributors are advised to maintain local inventory of high-turnover items to support clinic uptime. |
| 3. Does the installation of an excavator dental instrument require specialized technical support or on-site service? | Basic installation of modern excavator instruments can be performed by certified dental technicians; however, full system integration—especially with digital imaging or CAD/CAM workflows—requires trained field engineers. In 2026, manufacturers typically provide complimentary on-site installation and calibration for new units, including air pressure, water line, and electrical system verification. Remote diagnostic support is also standard for post-installation troubleshooting. |
| 4. What is covered under the standard warranty for excavator dental instruments, and what are the terms? | The standard warranty for excavator dental instruments in 2026 is 24 months from the date of commissioning, covering defects in materials and workmanship. This includes the motor assembly, control unit, and internal circuitry. Consumables (e.g., burs, tips) and damage from improper use or unauthorized modifications are excluded. Extended warranty options up to 5 years are available, including coverage for preventive servicing and software updates. |
| 5. How can clinics or distributors ensure continued warranty validity during the instrument’s lifecycle? | Warranty validity requires adherence to the manufacturer’s maintenance schedule, use of approved consumables and spare parts, and documentation of service records via the digital service log (accessible through the instrument’s IoT-enabled interface). Instruments must be installed and serviced exclusively by certified technicians. Distributors should register each unit upon delivery and maintain a digital service history for end-user clients to ensure uninterrupted warranty coverage. |
Need a Quote for Excavator Dental Instrument?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


