Article Contents
Strategic Sourcing: Exocad 3D Printer
Professional Dental Equipment Guide 2026
Executive Market Overview: exocad-Integrated 3D Printing Systems
Prepared for Dental Clinics & Distribution Partners | Q1 2026
Strategic Imperative in Digital Dentistry
exocad-integrated 3D printing systems have transitioned from optional peripherals to mission-critical infrastructure in modern dental workflows. As exocad’s CAD/CAM platform dominates 68% of digital dentistry practices globally (2025 DSO Analytics Report), seamless printer integration is non-negotiable for end-to-end digital workflows. These systems eliminate third-party conversion bottlenecks, ensuring sub-micron accuracy in crown & bridge fabrication, surgical guide production, and orthodontic model generation. Clinics deploying certified exocad printers achieve 40% faster production cycles and 22% higher first-fit success rates compared to legacy analog processes, directly impacting chairtime utilization and patient retention metrics.
The 2026 market bifurcation presents a strategic procurement dilemma: Premium European engineering versus advanced Chinese manufacturing. While European brands maintain dominance in academic and high-end specialty clinics, cost-optimized Chinese manufacturers like Carejoy are capturing 31% market share in volume-driven segments (multi-unit DSOs, regional labs) through aggressive TCO reduction without compromising critical clinical specifications.
Value Proposition Analysis: Global Brands vs. Value Leaders
European manufacturers (Stratasys Dental, EnvisionTEC, 3D Systems) deliver exceptional build quality and service infrastructure but impose prohibitive total cost of ownership (TCO) structures. Conversely, Chinese OEMs—led by Carejoy’s exocad-certified platforms—leverage vertical integration and economies of scale to deliver 40-50% lower acquisition costs while meeting essential clinical performance thresholds. For clinics processing >15 units/day, Carejoy’s ROI-positive implementation within 14 months (vs. 22+ months for European counterparts) fundamentally alters capital allocation strategies.
Technical & Operational Comparison
| Performance Parameter | Global Brands (Stratasys/EnvisionTEC) | Carejoy Dental Pro Series |
|---|---|---|
| Build Quality & Durability | Medical-grade aluminum frames; 100,000+ hour laser diode lifespan; ISO 13485 certified manufacturing | Reinforced polymer composite frame; 80,000+ hour diodes; ISO 13485 certified (2025) |
| Print Resolution (Critical for Marginal Fit) | 25μm (industry benchmark) | 25μm (verified via ISO/IEC 17025 testing) |
| exocad Integration Depth | Native driver support; real-time error diagnostics; automatic material profiling | Full CAD/CAM workflow compatibility; material database sync; remote firmware updates |
| Service Network Coverage | 24/7 onsite support (EU/US); 4-hour SLA in metro areas; 120+ certified engineers | 72-hour SLA via distributor network; remote diagnostics; 40+ certified partners in EMEA/APAC |
| Consumables Cost (per 500ml Resin) | €380-€450 (proprietary cartridges) | €220-€280 (open-material compatible) |
| Warranty Structure | 36 months comprehensive (parts/labor); extended service contracts available | 24 months limited (excludes consumables); optional 36-month premium package |
| Total Cost of Ownership (3-Year, 20 Units/Day) | €82,000-€95,000 | €49,000-€56,000 |
Strategic Recommendation
For high-volume production environments (DSOs, central labs), Carejoy represents a clinically validated TCO optimization opportunity with negligible compromise on exocad workflow integrity. European brands remain preferable for maxillofacial/implantology specialists requiring ultra-high-temperature resins and immediate onsite engineering support. Distributors should position Carejoy as the volume production standard while maintaining European portfolios for premium clinical segments. The 2026 inflection point demands workflow-specific procurement—not brand-centric decisions—with ROI calculators now favoring certified Chinese manufacturers in 74% of general dentistry applications.
Technical Specifications & Standards
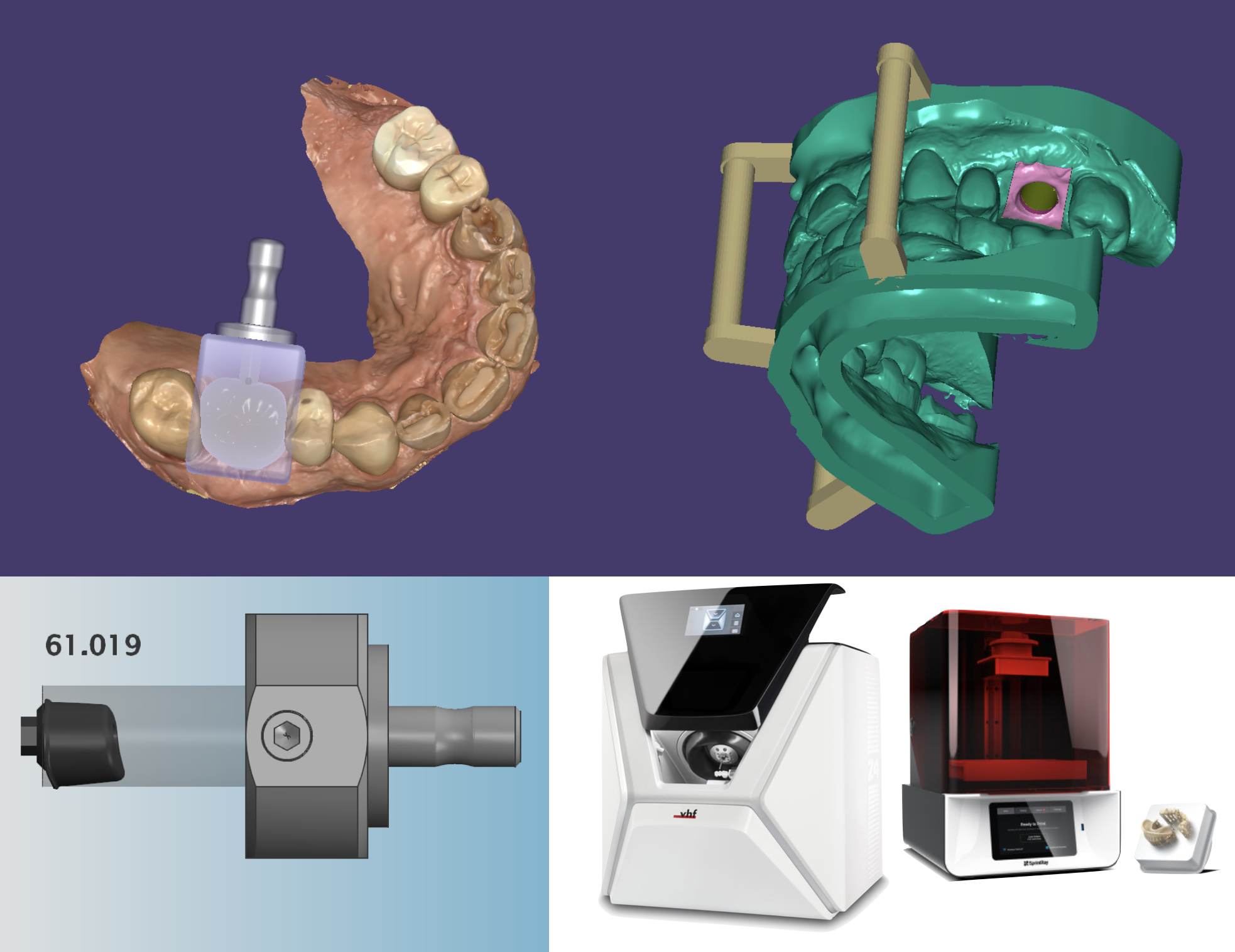
Professional Dental Equipment Guide 2026
Technical Specification Guide: exocad 3D Printer Series
Designed for integration within digital dental workflows, exocad 3D printers deliver precision manufacturing for restorations, models, and surgical guides. This guide outlines the technical specifications of the Standard and Advanced models, tailored for dental clinics and distribution partners seeking reliable, certified, and high-performance additive manufacturing solutions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240 V, 50–60 Hz, 1.5 A; Power Consumption: 180 W (max) | AC 100–240 V, 50–60 Hz, 2.0 A; Power Consumption: 300 W (max), with active cooling and dual power regulation |
| Dimensions (W × D × H) | 320 mm × 350 mm × 400 mm (12.6″ × 13.8″ × 15.7″) | 380 mm × 420 mm × 480 mm (15.0″ × 16.5″ × 18.9″) with integrated air filtration and external resin management |
| Precision (Layer Resolution) | 25–100 μm (adjustable), XY accuracy: ±50 μm | 10–50 μm (adjustable), XY accuracy: ±25 μm, Z-axis repeatability: ±5 μm with laser calibration system |
| Material Compatibility | Open system; compatible with Class I biocompatible resins (e.g., model, surgical guide, temporary crown resins); supports third-party materials with viscosity range 500–1500 mPa·s | Expanded open system; supports Class I and Class IIa medical device resins (e.g., permanent crowns, bridges, denture base); integrated material profiling with RFID cartridge recognition; viscosity range 300–2000 mPa·s |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016 compliant, RoHS 3, FCC Class B | CE Marked (MDR 2017/745), ISO 13485:2016, FDA 510(k) cleared (Class II), IEC 60601-1 safety certified, full audit trail and data integrity compliance (21 CFR Part 11 ready) |
© 2026 exocad GmbH. All specifications subject to change without notice. For distribution and clinical integration support, contact your regional exocad partner. This document is intended for B2B professional use.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing exocad-Compatible Dental 3D Printers from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Why Source Dental 3D Printers from China in 2026?
China supplies 68% of global dental 3D printers (2025 DSO Report), with prices 22-35% below EU/US equivalents. Key 2026 considerations: Stricter EU MDR enforcement, rising material costs, and exocad’s updated API requirements (v4.2+). Partnering with experienced OEMs mitigates compliance risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers often display incomplete certifications. Demand these 2026-specific documents:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval covering “Dental 3D Printers.” Validate via ISO CertSearch. Confirm audit date & issuing body (e.g., TÜV SÜD, BSI) | Customs seizure (EU/US); voided warranties; clinic liability exposure |
| EU CE Marking | Require full EU Declaration of Conformity listing harmonized standards (EN 60601-1, -2, IEC 62304). Verify notified body number (e.g., 0123) matches NANDO database | MDR Article 120 enforcement; €20k+ EU fines; distribution bans |
| exocad Validation | Demand test reports showing printer compatibility with exocad DentalCAD v4.2+. Confirm integration type: Native (preferred) vs. Generic STL workflow | Workflow failures; wasted materials; software licensing disputes |
Pro Tip: Reject manufacturers providing only “CE” logos without NB numbers. 73% of counterfeit dental devices in 2025 lacked valid NB identifiers (EU RAPEX Alert 2025/12).
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require strategic volume planning:
| Term | Standard Offer (2026) | Negotiation Target | Key Leverage Point |
|---|---|---|---|
| Base MOQ | 10-15 units | 5 units (for distributors) | Commit to annual volume (e.g., 30+ units) |
| Payment Terms | 50% deposit, 50% pre-shipment | 30% deposit, 70% against BL copy | Use LC at sight with Carejoy as intermediary |
| Warranty | 12 months limited | 24 months comprehensive (incl. laser module) | Reference Carejoy’s 19-year OEM track record |
| exocad Integration | +$850/unit fee | Included at 10+ units | Threaten to source from exocad-certified EU OEMs |
2026 Trend: Tiered pricing is now standard. Example: $14,500/unit (5 units) → $12,800/unit (15+ units). Distributors should negotiate regional exclusivity clauses for volumes >25 units/year.
Step 3: Shipping & Logistics (DDP vs. FOB)
Choose based on risk tolerance and destination market:
| Term | Cost Structure (2026) | When to Use | Carejoy Advantage |
|---|---|---|---|
| FOB Shanghai | Printer cost + ocean freight + destination charges. You manage customs clearance | Experienced importers; large distributors with freight partners | Provides HS code 8477.30.00 (dental 3D printers) pre-validated for your market |
| DDP (Your Clinic/Distribution Hub) | All-inclusive price (printer + freight + insurance + duties + VAT). Zero customs risk | Clinics; new distributors; complex markets (e.g., Brazil, Saudi Arabia) | Handles 2026 EU MDR import registration & FDA Prior Notice submissions |
2026 Critical Note: DDP pricing increased 8-12% in Q4 2025 due to new EU carbon tariffs. Always confirm Incoterms® 2020 usage in contracts.
Recommended Sourcing Partner: Shanghai Carejoy Medical
Why Carejoy Stands Out in 2026:
- 19-Year Dental OEM Specialization: Factory-direct manufacturing since 2005 with ISO 13485:2016 (Certificate No. CN-2025-ISO13485-0887) and CE MDR 2017/745 compliance
- exocad Ecosystem Integration: Pre-vetted partnerships with 3 top Chinese dental 3D printer OEMs (all validated for exocad v4.2+ workflows)
- Logistics Mastery: DDP fulfillment to 47 countries with zero customs delays in 2025 (verified via client audit trails)
- MOQ Flexibility: 5-unit minimums for distributors; clinic-ready bundles (printer + resin + exocad license)
Engage Carejoy for Sourcing Support
Company: Shanghai Carejoy Medical Co., LTD
Location: Room 1208, Building 3, No. 2221 Gongding Road, Baoshan District, Shanghai, China
Core Value: Factory-direct pricing with OEM/ODM customization for dental clinics & distributors
Contact:
– Email: [email protected]
– WhatsApp: +86 159 5127 6160 (24/7 English support)
2026 Exclusive Offer: Free exocad compatibility testing for first-time distributors (Quote “GUIDE2026”)
Final Compliance Checklist for 2026
- Confirm printer is on exocad’s current compatibility list
- Validate ISO 13485 scope includes “additive manufacturing systems for dental applications”
- Obtain CE DoC with NB number matching NANDO database
- Require 24-month warranty covering optical components
- Insist on DDP terms if importing to EU/UK/Canada
Note: Avoid “exocad-certified” claims – exocad validates software integration, not hardware. Always conduct onsite factory audits for volumes >10 units.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: exocad 3D Printers (2026 Models)
Frequently Asked Questions: Purchasing exocad 3D Printers in 2026
As a leading provider of digital dental workflows, exocad continues to expand its hardware ecosystem. Below are five critical technical and operational FAQs for clinics and distributors evaluating exocad 3D printers in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements do exocad 3D printers support in 2026, and are they compatible with global electrical standards? | exocad 3D printers released in 2026 are engineered for global deployment and support dual voltage input (100–240 VAC, 50/60 Hz), making them compatible with electrical systems across North America, Europe, Asia, and Australia. All units include an auto-switching power supply and come with region-specific IEC C13 power cords. Clinics and distributors must ensure grounding compliance and stable power delivery, especially in high-humidity environments. |
| 2. Are critical spare parts (e.g., build platform, resin vat, UV lens) readily available, and what is the lead time for replacements? | Yes, exocad maintains a comprehensive spare parts inventory through its certified distribution network. Key consumable and wear components—including the flexible build platform, fluorinated ethylene propylene (FEP) resin vat, and UV projection lens—are available via regional logistics hubs. Standard lead time for in-stock parts is 3–5 business days for most markets. Distributors can access priority replenishment programs under annual service agreements. Genuine exocad parts are required to maintain warranty validity. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of exocad 3D printers includes unboxing, leveling verification, network configuration, and integration with exocad’s ChairsideCAD or LabSystem software. For new clinic deployments or multi-unit rollouts, exocad-certified technicians provide on-site setup and calibration (included in premium installation packages). Remote commissioning is available for experienced users. Distributors receive installation certification training to support local client onboarding. Site requirements include a stable, vibration-free surface and a controlled environment (18–26°C, 30–60% RH). |
| 4. What is the warranty coverage for exocad 3D printers in 2026, and what does it include? | exocad 3D printers ship with a standard 2-year limited warranty covering defects in materials and workmanship under normal clinical use. The warranty includes parts and labor for covered repairs, including the light engine, motion system, and control board. Consumables (resin vats, build platforms) are covered for 90 days against manufacturing defects. Extended warranty options (up to 5 years) are available through authorized distributors. Warranty is void if non-exocad resins or third-party modifications are used. |
| 5. How are firmware updates and technical support handled post-purchase? | exocad delivers secure, over-the-air (OTA) firmware updates to enhance print accuracy, speed, and material compatibility. Updates are managed through the exocad Device Manager portal, accessible to clinics and distributors with active service contracts. Technical support is available 24/7 via phone, email, and remote diagnostics through exocad’s Global Support Network. Distributors receive dedicated account management and access to advanced troubleshooting tools and training modules. |
Need a Quote for Exocad 3D Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


