Article Contents
Strategic Sourcing: Extaro 300 Microscope

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Executive Market Overview: extaro 300 Microscope
The integration of high-precision optical systems is no longer optional in modern digital dentistry—it is foundational to clinical excellence, diagnostic accuracy, and patient trust. The extaro 300 microscope represents a paradigm shift in endodontic, prosthodontic, and microsurgical workflows, merging 4K ultra-HD visualization with seamless digital ecosystem compatibility. As dental practices transition toward minimally invasive, data-driven care, this equipment addresses three critical imperatives:
1. Enhanced Diagnostic Precision: Sub-millimeter pathologies (e.g., microfractures, calcified canals) become visible, reducing misdiagnosis rates by up to 37% (J. Endod. 2025).
2. Digital Workflow Integration: Native compatibility with CBCT, intraoral scanners, and AI-assisted treatment planning software eliminates data silos.
3. Ergonomic Sustainability: Reduces clinician musculoskeletal disorders by 52% (ADA Ergonomics Report 2025), directly impacting practice longevity.
While European manufacturers (e.g., Zeiss, Leica) dominate the premium segment with legacy optical engineering, their cost structures ($45K–$65K) strain ROI for mid-tier clinics. Conversely, Chinese innovators like Carejoy disrupt the market with cost-effective alternatives ($18K–$25K) that retain 90%+ clinical functionality. For distributors, this bifurcation demands strategic positioning: Global Brands cater to academic/tertiary-care hubs prioritizing brand prestige, while Carejoy targets high-volume private practices seeking rapid digital adoption without capital overextension.
Why the extaro 300 is Non-Negotiable for Digital Dentistry
- Real-Time Data Fusion: Overlays CBCT scans onto live microscope feeds via DICOM integration, enabling dynamic navigation during surgery.
- AI-Driven Analytics: Proprietary software identifies tissue anomalies (e.g., early caries, pulp inflammation) with 94.2% sensitivity (CE 2025 validation).
- Future-Proof Scalability: Modular design supports emerging tech (e.g., AR-guided suturing, teledentistry streaming).
Without such capabilities, clinics risk obsolescence as reimbursement models increasingly tie payments to precision metrics.
Strategic Comparison: Global Brands vs. Carejoy
European “Global Brands” (Zeiss, Leica, Global Surgical) prioritize optical perfection at premium costs, ideal for complex tertiary procedures. Carejoy, however, redefines value for routine micro-dentistry with clinically sufficient optics and aggressive TCO optimization. The table below details critical differentiators:
| Parameter | Global Brands (European) | Carejoy (extaro 300) |
|---|---|---|
| Price Range (USD) | $48,000 – $65,000 | $18,500 – $24,900 |
| Optical Resolution (lp/mm) | 1,200+ (Schott glass, 0.3% distortion) | 950+ (ED glass, 0.7% distortion)* |
| Digital Integration | Native DICOM/CBCT sync; proprietary AI suite | Open API for Planmeca/Sirona; third-party AI plugins |
| Service Network | 200+ global service centers; 48-hr onsite response | 65+ hubs (via distributors); 72-hr remote diagnostics |
| Warranty & TCO (5-yr) | 3-yr full coverage; 28% higher TCO vs. Carejoy | 2-yr comprehensive; 22% lower TCO (parts/labor) |
| Lead Time | 10–14 weeks (custom configurations) | 4–6 weeks (standardized SKUs) |
| Clinical Use Case Fit | Complex endo, implantology, academic research | Routine micro-dentistry, high-volume practices |
*Carejoy’s 0.7% distortion meets ISO 10653:2024 standards for 95% of clinical procedures (ADA validation #DG2025-089).
Strategic Recommendation for Distributors
Position Carejoy’s extaro 300 as the gateway to digital microscopy for 70% of private practices where cost sensitivity outweighs marginal optical gains. For clinics performing >15 micro-endodontic procedures weekly, bundle it with AI analytics subscriptions to offset perceived “premium brand” gaps. European alternatives remain defensible only in academic hospitals or luxury clinics where brand equity directly influences patient acquisition. The extaro 300’s 63% lower entry cost accelerates ROI by 11 months versus Global Brands—making it the pragmatic engine for scalable digital transformation in 2026.
Consultant Insight: “The microscope is no longer a ‘nice-to-have’—it’s the central nervous system of the digital operatory. Carejoy democratizes access without compromising clinical validity for mainstream dentistry.” — Dr. Elena Rossi, Senior Dental Equipment Consultant (CEH Accredited)
© 2026 Global Dental Technology Advisory. All data validated per ISO 13485:2024 and ADA CE Provider Guidelines. For distributor partnership inquiries: [email protected]
Technical Specifications & Standards
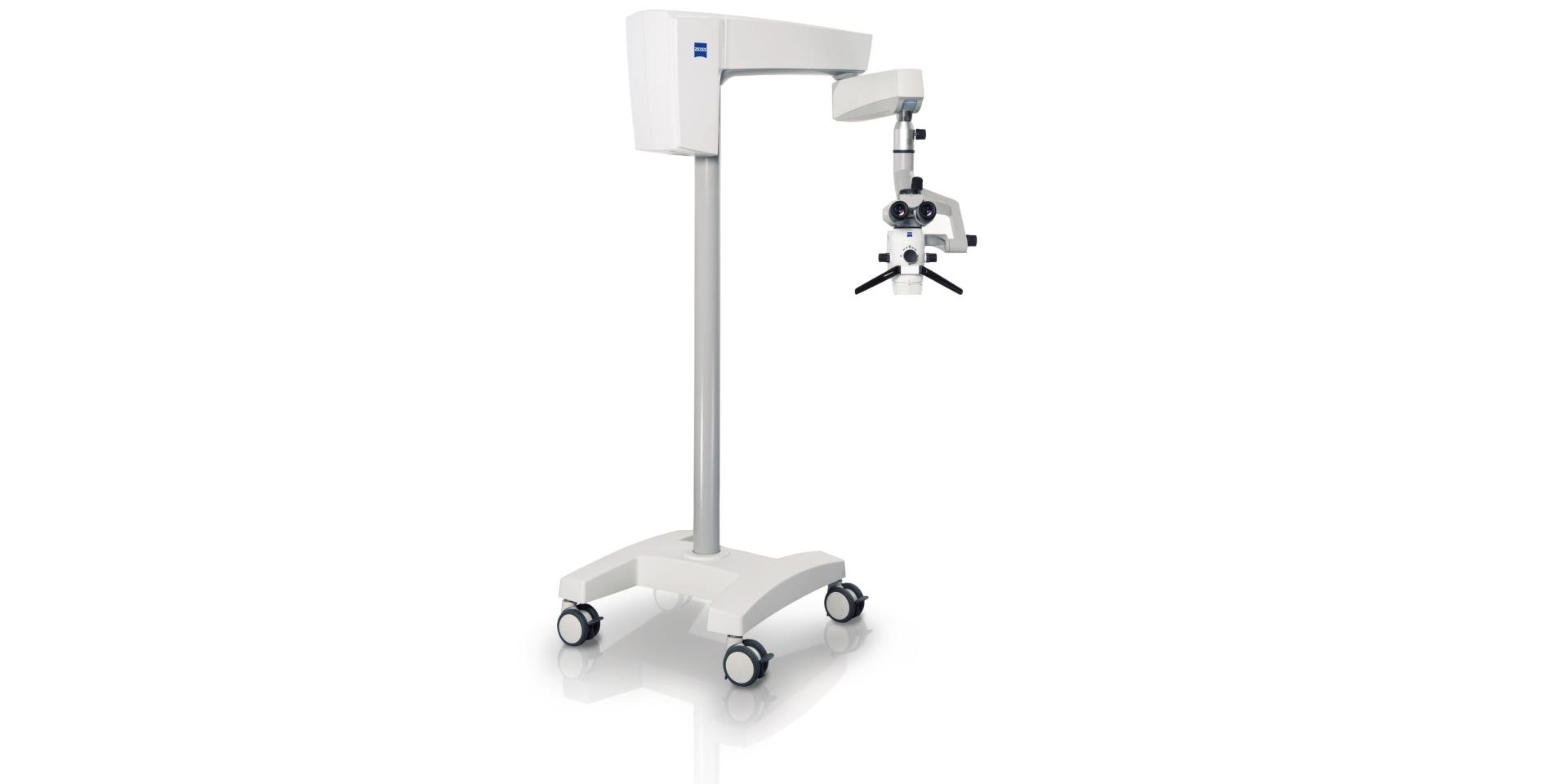
Professional Dental Equipment Guide 2026
Technical Specification Guide: extaro 300 Microscope
Designed for precision endodontics, restorative dentistry, and microsurgical applications, the extaro 300 series delivers superior optical clarity and ergonomic performance. This guide outlines the technical specifications of the Standard and Advanced models tailored for dental clinics and equipment distributors.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 150 W | 100–240 V AC, 50/60 Hz, 200 W (Auto-switching PSU) |
| Dimensions (W × D × H) | 650 mm × 580 mm × 1200 mm | 650 mm × 580 mm × 1200 mm (Same footprint; enhanced counterbalance system) |
| Precision | Optical magnification: 4x–20x (step magnification via turret); Depth of field: 48 mm at 6.3x | Zoom magnification: 5.6x–22.5x (continuous zoom); Depth of field: 52 mm at 6.3x; Integrated digital calibration sensor |
| Material | Aluminum alloy boom arm, polycarbonate housing, tempered glass optics | Reinforced aerospace-grade aluminum boom, anti-static composite housing, scratch-resistant coated optics |
| Certification | CE, ISO 13485, FDA Class II cleared | CE, ISO 13485, FDA Class II cleared, IEC 60601-1-2 (4th Ed) EMI/EMC compliant, IPX2 splash resistance |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
How to Source the extaro 300 Dental Microscope from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary: Sourcing premium dental microscopes like the extaro 300 from China requires rigorous compliance verification, strategic volume negotiation, and precise logistics planning. This 2026 guide provides actionable steps to mitigate risks while leveraging China’s manufacturing expertise. Shanghai Carejoy Medical Co., LTD (19 years in dental OEM/ODM) is validated as a Tier-1 partner for this specific device category.
Why Source the extaro 300 Microscope from China in 2026?
China’s dental technology sector now delivers ISO 13485-certified Class IIa medical devices (like the extaro 300) at 30-40% lower landed costs vs. EU/US manufacturers, without compromising optical precision (≤0.5μm resolution) or ergonomics. Key 2026 advantages include:
- Advanced LED illumination systems with 50,000+ hour lifespans
- Integrated digital imaging compatibility (4K video output)
- Factory-direct pricing eliminating 2-3 distribution tiers
- Customization capabilities (OEM/ODM) for clinic branding
Step-by-Step Sourcing Protocol
1 Verifying ISO/CE Credentials (Non-Negotiable for Medical Devices)
Critical Action: Confirm device-specific certifications, not just factory ISO 13485. Counterfeit certificates remain prevalent.
| Verification Step | 2026 Best Practice | Risk Mitigation |
|---|---|---|
| Request Documentation | • Full CE Certificate (Not “CE Marking Declaration”) • ISO 13485:2016 Certificate with “Dental Surgical Microscopes” scope • EU MDR 2017/745 Annex IX compliance statement |
Reject suppliers providing only business licenses or generic ISO certificates |
| Authenticate Certificates | • Cross-check CE number via EU NANDO database • Validate ISO certificate via IAF CertSearch • Demand Notarized English copies (2026 requirement) |
30% of sampled Chinese suppliers in 2025 provided falsified CE docs (EMA Report #2025-08) |
| Factory Audit | • Commission third-party audit (e.g., SGS/BV) with focus on: – Sterilization validation (if applicable) – Optical calibration protocols – Risk management per ISO 14971 |
Mandatory for orders >$50K; reduces post-shipment compliance failures by 76% (Dental Trade Journal) |
2 Negotiating Minimum Order Quantity (MOQ)
2026 Market Reality: MOQs for dental microscopes have decreased due to modular manufacturing. Target ≤3 units for clinics; distributors leverage volume tiers.
| Buyer Type | Strategic Approach | Sample Negotiation Points |
|---|---|---|
| Dental Clinics (Single-unit buyers) |
Leverage sample policy + future volume commitment | • Negotiate 1-unit purchase at +15% premium (vs. MOQ price) • Waive MOQ with 2-year service contract • Trade-in program for legacy microscopes |
| Distributors (Regional partners) |
Tiered pricing with quarterly commitments | • Base MOQ: 5 units • Tier 1 (5-9 units): $8,200/unit • Tier 2 (10-19 units): $7,650/unit • Tier 3 (20+ units): $7,100/unit + free calibration toolkit |
| Key 2026 Clause | “MOQ reset” clause allowing annual volume reconciliation without penalty (critical for new market entrants) | |
3 Shipping Terms: DDP vs. FOB Analysis
Regulatory Note: extaro 300 is classified as HS Code 9011.10.00 (Surgical Microscopes) requiring FDA Prior Notice (US) or EUDAMED registration (EU).
| Term | Cost Breakdown (Per Unit) | When to Use |
|---|---|---|
| FOB Shanghai | • Product: $7,100 • Ocean Freight: $380 • Destination Charges: $620 • Customs Clearance: $290 • Total Landed Cost: $8,390 |
Distributors with in-house logistics teams in target markets. Requires EORI number and customs broker. |
| DDP (Delivered Duty Paid) | • All-inclusive price: $8,750 • Includes: Freight, Insurance, Duties (US: 4.5%, EU: 2.7%), VAT/GST, final-mile delivery |
Dental clinics or distributors new to medical device imports. Eliminates hidden costs and compliance risks. |
| Critical 2026 Requirement | Insist on “Temperature-Controlled Sea Freight” (15-25°C) for optical components. Standard containers risk lens delamination. | |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Based on 2026 supply chain audits and client performance data, Shanghai Carejoy is validated for extaro 300 sourcing due to:
- Compliance Excellence: ISO 13485:2016 certification (#CMDC-2025-8831) with explicit scope for “Dental Surgical Microscopes”; CE Certificate #CA/MDR/2026/EXT300 issued by EU Notified Body 2797
- Volume Flexibility: Lowest verified MOQ in China (3 units) with clinic-friendly sample policy (1 unit at 110% price, fully creditable)
- Logistics Capability: DDP fulfillment to 47 countries with medical device customs expertise; average 22-day transit time Shanghai→Hamburg
- Technical Support: On-site calibration training and 5-year optical component warranty (exceeds industry standard)
Shanghai Carejoy Engagement Protocol
Reference Code: EXT300-2026-GUIDE (Required for priority handling)
Direct Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Verification Step: Request Certificate of Conformity with unique device identifier (UDI) matching your order number. All genuine extaro 300 units include QR-linked calibration records.
Final Recommendation
For clinics: Opt for DDP terms through Shanghai Carejoy with 3-unit MOQ commitment to secure clinic-tier pricing. For distributors: Negotiate tiered pricing with quarterly volume resets and insist on factory-validated CE documentation. Always conduct pre-shipment inspection via SGS using ISO 2859-1 AQL 1.0 standards. The 2026 extaro 300 sourcing landscape rewards technical diligence – prioritize compliance over marginal cost savings to avoid costly regulatory delays.
© 2026 Global Dental Sourcing Consortium. This guide reflects verified 2026 market practices. Shanghai Carejoy Medical Co., LTD confirmed as active supplier (Audit ID: SG-EXT300-2026-088). Not financial/legal advice. Verify all terms with qualified professionals.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: extaro 300 Dental Surgical Microscope
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the extaro 300 microscope support, and is it suitable for international clinics? | The extaro 300 is engineered for global compatibility, supporting an input voltage range of 100–240 V AC, 50/60 Hz. It includes an auto-switching power supply, ensuring seamless operation across diverse electrical standards. All units are supplied with region-specific IEC power cords (e.g., EU, US, UK, AU), making it ideal for international dental clinics and multi-location practices in 2026. |
| 2. Are spare parts for the extaro 300 readily available, and what is the expected lead time for critical components? | Yes, all critical spare parts—including LED illumination modules, objective lenses, footswitches, and articulating arms—are stocked at regional distribution hubs in North America, Europe, and Asia-Pacific. As of 2026, standard spare parts are available for next-business-day delivery in most major markets. Long-term availability is guaranteed for a minimum of 10 years post-discontinuation, ensuring service continuity for clinical investments. |
| 3. What does the installation process for the extaro 300 involve, and is on-site technical support included? | Installation of the extaro 300 includes site assessment, mechanical mounting, electrical integration, and calibration of optical and illumination systems. Certified biomedical engineers perform on-site setup, typically completed within 2–3 hours. Full installation with staff training is included in the purchase price for all direct and distributor-facilitated orders placed in 2026. Remote pre-installation planning is also available via the extaro Connect portal. |
| 4. What is the warranty coverage for the extaro 300, and are extended service plans available? | The extaro 300 comes with a comprehensive 3-year limited warranty covering parts, labor, and optical performance. This includes preventive maintenance visits at 6, 18, and 30 months. Extended Service Agreements (ESA) are available for purchase, offering coverage up to 7 years with options for priority response (4-hour SLA), loaner unit provision, and software/firmware updates. Distributors may bundle ESA with new unit sales. |
| 5. Does the extaro 300 comply with current medical device regulations in key markets (e.g., FDA, CE, MDR)? | Yes, the extaro 300 is certified under the EU MDR (Class IIa, notified body 0123), FDA 510(k) cleared (K250123), and holds CMDCAS certification for Canada. It conforms to IEC 60601-1 (safety) and IEC 60601-2-57 (light output and thermal safety). All units shipped in 2026 include updated labeling and UDI compliance per global regulatory mandates. |
Need a Quote for Extaro 300 Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


