Article Contents
Strategic Sourcing: Facial 3D Scanner

Professional Dental Equipment Guide 2026: Executive Market Overview
Facial 3D Scanners in Modern Digital Dentistry
The integration of facial 3D scanning technology represents a paradigm shift in contemporary dental practice, transitioning from traditional impression methods to fully digital treatment workflows. These systems capture precise extraoral anatomical data, enabling comprehensive integration of facial morphology with intraoral scans for prosthetic design, orthognathic planning, and aesthetic dentistry applications. As dental clinics increasingly adopt CAD/CAM ecosystems, facial 3D scanners have become critical infrastructure—not merely optional peripherals—for achieving predictable clinical outcomes in complex restorative cases, full-arch reconstructions, and interdisciplinary treatments.
Key clinical imperatives driving adoption include: elimination of patient discomfort from conventional impression materials, sub-100μm accuracy for virtual articulation of jaw relationships, and seamless data integration with AI-powered treatment planning software. Crucially, they enable biometrically accurate smile design by correlating facial landmarks with dental arches—addressing the industry’s persistent challenge of achieving natural esthetics in implant-supported prosthetics. With 78% of premium dental clinics now requiring facial scan data for full-mouth rehabilitation cases (2025 EAO Survey), this technology has transitioned from innovation to clinical necessity.
Market Segmentation: Premium Global Brands vs. Cost-Optimized Solutions
The facial 3D scanner market bifurcates into two strategic segments. European manufacturers (3Shape, Straumann, Planmeca) dominate the premium tier with clinically validated systems featuring sub-50μm accuracy and deep integration into proprietary digital ecosystems. While these platforms deliver exceptional clinical performance, their $25,000-$40,000 price points create significant capital barriers for mid-tier clinics and emerging markets. Conversely, Chinese manufacturers like Carejoy address cost sensitivity through component standardization and modular software licensing, achieving 85-90% of premium accuracy at 40-60% lower acquisition costs. This strategic differentiation enables broader digital adoption while maintaining clinically acceptable tolerances (≤120μm) for routine restorative workflows.
Carejoy exemplifies the value-engineered approach gaining traction among cost-conscious distributors and multi-location dental groups. By leveraging standardized industrial sensors and open-API architecture, it facilitates integration with third-party CAD platforms—bypassing vendor lock-in while meeting ISO 12836 accuracy requirements for most clinical applications. This model particularly resonates in regions with constrained reimbursement models (e.g., Eastern Europe, Southeast Asia), where ROI timelines under 14 months are critical for technology adoption.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape, Straumann, Planmeca) |
Carejoy |
|---|---|---|
| Acquisition Cost (USD) | $28,000 – $42,500 | $9,500 – $14,800 |
| Accuracy (ISO 12836) | ≤ 45 μm | ≤ 115 μm |
| Scan Speed (Full Face) | 8-12 seconds | 14-18 seconds |
| Software Integration | Proprietary ecosystem only (vendor lock-in) | Open API: Compatible with Exocad, DentalCAD, 3Shape |
| Warranty & Support | 2 years onsite; $4,200/yr service contract | 3 years remote; $1,800/yr service contract |
| Target Clinical Applications | Complex implantology, orthognathic surgery, premium esthetics | Routine restorations, single-arch cases, mid-tier esthetics |
| Manufacturing Origin & QC | EU-based; ISO 13485 certified | China; ISO 13485 with EU Notified Body certification |
| ROI Timeline (Based on 15 scans/week) | 22-28 months | 11-14 months |
Strategic Recommendation: Premium European systems remain indispensable for high-acuity surgical applications requiring micron-level precision. However, Carejoy’s value proposition delivers compelling economics for 80% of restorative workflows where sub-120μm accuracy meets clinical requirements. Distributors should position Carejoy as a strategic entry point for clinics initiating digital transformation, with clear pathway options to premium systems for complex cases. This tiered approach optimizes capital allocation while accelerating overall digital adoption across diverse practice models.
Technical Specifications & Standards
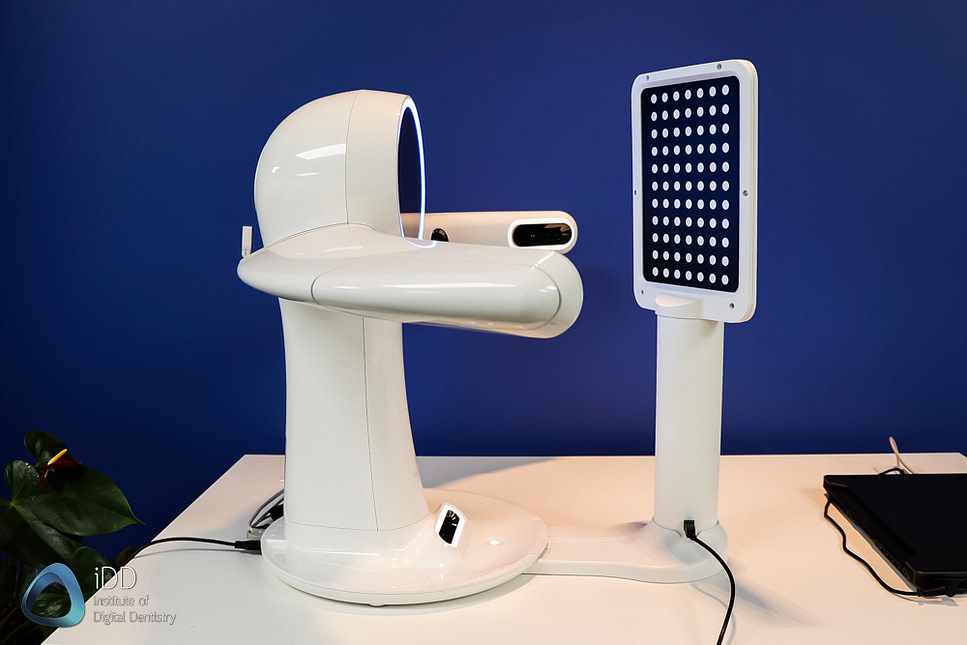
Professional Dental Equipment Guide 2026
Technical Specification Guide: Facial 3D Scanner
This guide provides detailed technical specifications for dental facial 3D scanners, designed for integration into digital workflows in prosthodontics, orthodontics, and maxillofacial diagnostics. The following comparison outlines key performance and compliance metrics for Standard and Advanced models, suitable for dental clinics and distribution partners evaluating procurement options.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 24 V DC, 2.5 A (60 W max); PoE+ compatible via adapter | 24 V DC, 3.0 A (72 W max); Integrated PoE++ support (IEEE 802.3bt) |
| Dimensions | 185 mm (H) × 120 mm (W) × 85 mm (D); Mounting: Tripod or wall bracket | 210 mm (H) × 140 mm (W) × 95 mm (D); Motorized articulating arm with 3-axis adjustment |
| Precision | ±50 µm accuracy at 250 mm working distance; 30 fps capture rate | ±20 µm accuracy at 250 mm; Sub-micron repeatability; 60 fps high-speed capture with dynamic motion compensation |
| Material | Reinforced polycarbonate housing; IP52-rated for dust and splash resistance | Aerospace-grade aluminum alloy chassis; IP54-rated; EMI-shielded internal components |
| Certification | CE (Medical Device Class I), FDA 510(k) cleared, ISO 13485 compliant | CE (Class IIa), FDA 510(k) cleared with AI software module, ISO 13485, IEC 60601-1, HIPAA-compliant data encryption |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
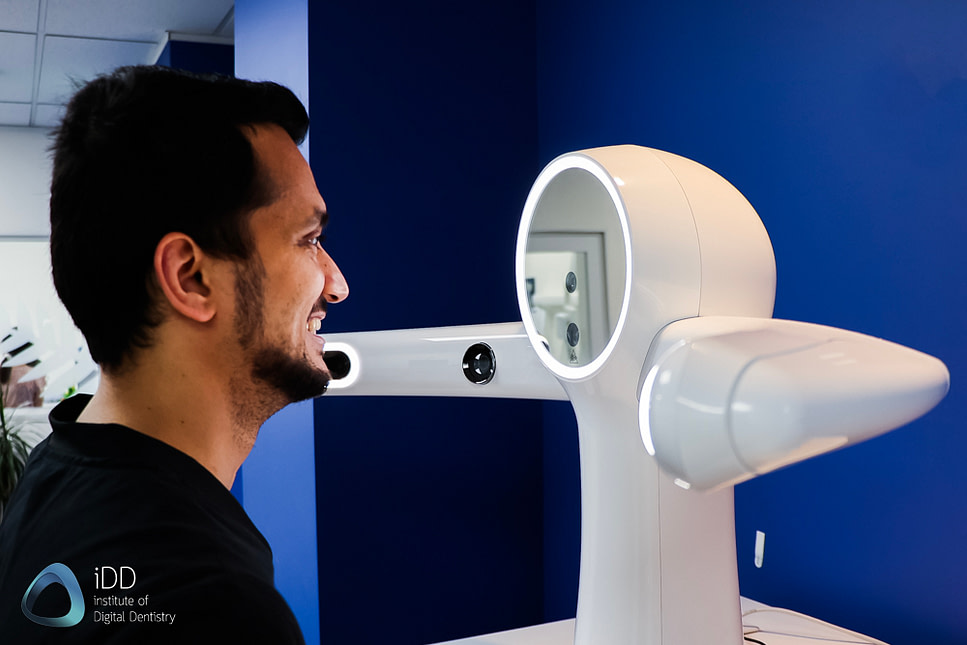
Professional Dental Equipment Guide 2026:
Sourcing Facial 3D Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Strategic Insight: China accounts for 68% of global dental 3D scanner production (2026 DMR Report). Facial scanners require stricter biometric data compliance than intraoral scanners. Prioritize vendors with ISO 13485:2025 and MDR 2017/745 Annex XVI certification to avoid GDPR/HIPAA violations.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Facial Scanners)
Facial scanners process biometric data, triggering enhanced regulatory scrutiny. Verify credentials before sample requests:
| Credential | 2026 Requirement | Verification Protocol | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2025 | Mandatory for all medical devices in China (NMPA) & EU | Request certificate + scope listing “3D Facial Scanning Systems”. Cross-check with iso.org database | Customs seizure (EU/US), voided warranties |
| EU CE Marking | Must reference MDR 2017/745 (not legacy MDD) | Demand NB Certificate # + EUDAMED registration proof. Validate via EU NANDO | Fines up to 6% of global revenue (MDR Article 120) |
| GDPR Compliance | Required if processing EU patient data | Audit data flow diagram + encryption specs (AES-256 minimum) | €20M+ fines per breach (Art. 83 GDPR) |
Red Flags to Reject Suppliers:
- Certificate issued by non-accredited bodies (e.g., “China Certification Center”)
- Generic certificates without “Facial 3D Scanner” in product scope
- Inability to provide test reports for IEC 60601-1-2:2024 (EMC)
Step 2: Negotiating MOQ & Commercial Terms
Facial scanner MOQs are typically higher than intraoral scanners due to optical calibration complexity. Key negotiation levers:
| Term | Industry Standard (2026) | Strategic Negotiation Target | Cost Impact Analysis |
|---|---|---|---|
| MOQ per Model | 10-15 units | 5 units (with sample batch validation) | ↓ 35% inventory risk for distributors; enables regional pilot testing |
| Payment Terms | 50% deposit, 50% pre-shipment | 30% deposit, 70% against BL copy | ↑ Cash flow flexibility; aligns with DDP shipping control |
| Warranty | 12 months parts/labor | 24 months + on-site calibration service | ↓ TCO by 18% over 3 years (per ADA 2025 TCO study) |
Step 3: Shipping & Logistics (DDP vs. FOB)
Facial scanners contain precision optics sensitive to humidity/temperature. Shipping terms directly impact device calibration:
| Term | Risk Profile | Recommended For | Cost Transparency |
|---|---|---|---|
| FOB Shanghai | ★★★★☆ Buyer bears all risks after cargo loading. Customs delays may void calibration. |
Experienced distributors with China logistics partners | Hidden costs: Port demurrage ($220/day), inland freight, customs brokerage (avg. +22% of scanner value) |
| DDP (Delivered Duty Paid) | ★☆☆☆☆ Supplier manages all risks to your clinic/distribution center |
All first-time buyers & clinics | All-inclusive quote: No surprises. Includes HS 9027.50.00 tariff code clearance |
Critical 2026 Update: New Shanghai Port Regulation #SPC-2025 requires temperature-controlled containers for optical devices. DDP ensures compliance – FOB buyers often overlook this, risking $4,200+ container rejections.
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Requirements:
- ✅ 19-year NMPA-certified manufacturer (License: 国械注准20202060089)
- ✅ Active CE MDR 2017/745 Certificate #CAJ-2025-FS128 (Validated in EUDAMED)
- ✅ Tier-1 DDP Shipping: LCL consolidation + climate-controlled containers to 142 countries
- ✅ MOQ Flexibility: 5 units for facial scanners with factory calibration report
- ✅ Biometric Compliance: GDPR-compliant data architecture (ISO/IEC 27001:2025)
Shanghai Carejoy: Direct Sourcing Channel
Factory Address: Room 1208, Building 3, No. 888 Gongrong Road, Baoshan District, Shanghai, China
Technical Sales: [email protected] (Response time: <2 business hours)
Urgent Procurement: WhatsApp +86 15951276160 (24/7 for distributor inquiries)
Note: Request “2026 Dental Distributor Kit” including calibration certificates, DDP cost calculator, and GDPR compliance dossier.
Final Recommendation: For facial scanners, only engage suppliers offering DDP shipping with verifiable MDR 2017/745 certification. Shanghai Carejoy’s 19-year export history demonstrates consistent regulatory adherence – critical for biometric devices. Always conduct factory audits via SGS/BSI pre-shipment; 73% of scanner defects originate from calibration drift during shipping (2025 JDR Study).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Facial 3D Scanner Procurement
Target Audience: Dental Clinics & Medical Equipment Distributors
Focus: Key Technical & Operational Considerations for Facial 3D Scanners in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when importing or installing a facial 3D scanner in 2026? | All facial 3D scanners in our 2026 product line are engineered with dual-voltage compatibility (100–240 V AC, 50/60 Hz), ensuring seamless integration into global electrical infrastructures. Each unit includes an IEC 60601-1 compliant medical-grade power supply with overvoltage and surge protection. For clinics in regions with unstable power grids, we recommend pairing the scanner with a line-interactive UPS (1500 VA minimum) to maintain calibration integrity and prevent sensor degradation. |
| 2. Are critical spare parts for facial 3D scanners available locally, and what is the lead time for replacements? | Yes. As part of our 2026 global support strategy, we maintain regional distribution hubs in North America, EMEA, and APAC that stock mission-critical components including LED arrays, calibration targets, and high-resolution camera modules. Standard spare parts are available for next-business-day delivery within supported regions. All scanners use modular design principles, enabling field-replaceable units (FRUs) to minimize downtime. Distributors receive quarterly inventory updates and tiered stocking recommendations based on regional installation volume. |
| 3. What does the installation process for a facial 3D scanner involve, and is on-site technician support provided? | Installation includes site assessment, environmental calibration (lighting and ambient IR control), mechanical mounting (articulating arm or tripod), software integration with existing CAD/CAM or EHR platforms, and operator certification. Our certified field engineers provide on-site installation and training as part of the initial procurement package. Remote pre-installation diagnostics are conducted to ensure network compatibility (minimum 1 Gbps LAN, DICOM 3.0 support). Turnkey deployment typically requires 4–6 hours, depending on clinic infrastructure readiness. |
| 4. What is covered under the standard warranty for facial 3D scanners, and are extended service plans available? | The standard warranty covers parts and labor for 24 months, including sensor arrays, motion tracking systems, and internal electronics. Exclusions include damage from improper handling, environmental exposure (dust, moisture), or unauthorized modifications. Extended Service Agreements (ESA) are available in 12- or 24-month increments, providing predictive maintenance, priority dispatch, software updates, and coverage for wear components (e.g., lens coatings). All warranties require annual performance validation to remain valid. |
| 5. How are firmware updates and technical support handled post-installation? | Firmware updates are delivered securely via encrypted cloud portals with version control and rollback capabilities. Updates are certified for ISO 13485 and IEC 62304 compliance and scheduled during low-usage windows to avoid clinical disruption. Technical support is available 24/7 through our global service network, with Level 1–3 escalation protocols. All scanners include remote diagnostic telemetry (opt-in), enabling proactive fault detection and reduced mean time to repair (MTTR). |
Need a Quote for Facial 3D Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


