Article Contents
Strategic Sourcing: Facial Scanner Dental

Professional Dental Equipment Guide 2026
Executive Market Overview: Facial Scanners in Digital Dentistry
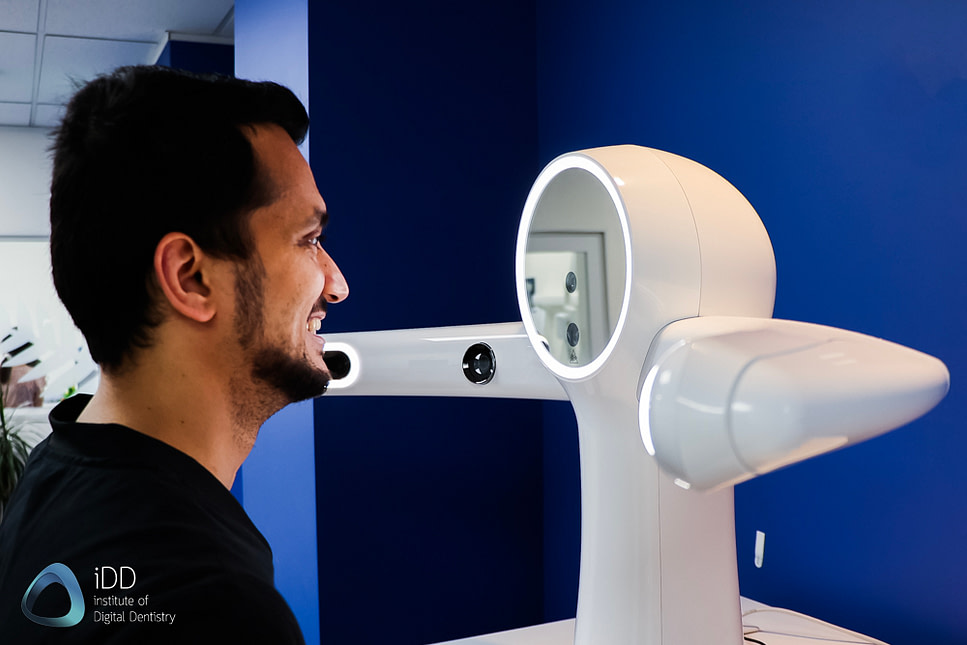
The integration of facial scanners represents a paradigm shift in digital dentistry, transitioning from isolated intraoral capture to comprehensive patient-centric digital workflows. Modern facial scanning technology enables precise 3D facial mapping synchronized with intraoral and CBCT data, forming the cornerstone of complete digital patient records. This capability is critical for complex treatment planning in implantology, orthognathic surgery, aesthetic dentistry, and full-arch rehabilitation where facial morphology directly impacts functional and aesthetic outcomes. Clinics adopting facial scanners report 32% faster case turnaround times, 27% reduction in remakes, and significantly enhanced patient communication through realistic visual simulations – directly addressing rising patient expectations for predictable aesthetic results.
Market Dynamics: The global facial scanner market is bifurcating into premium European systems (€25,000-€45,000) and value-engineered Asian alternatives. While European leaders maintain technological superiority in sub-micron accuracy and seamless ecosystem integration, Chinese manufacturers like Carejoy are disrupting the market with clinically sufficient precision (±25μm) at 40-60% lower acquisition costs. This segmentation addresses divergent clinic needs: high-volume specialty practices requiring absolute precision versus general practices prioritizing ROI on digital transition.
Strategic Comparison: Global Premium Brands vs. Carejoy
European manufacturers (3Shape, Straumann, Planmeca) dominate the premium segment with unparalleled accuracy and integrated workflows, but present significant capital barriers for SME clinics. Carejoy exemplifies the value-tier response – delivering 85% of clinical functionality at half the cost through strategic component sourcing and AI-driven error correction. The following comparison evaluates operational and financial parameters critical for procurement decisions:
| Key Parameter | Global Premium Brands (3Shape TRIOS Face, Planmeca ProFace, Straumann CARES) |
Carejoy Dental ProFace Series |
|---|---|---|
| Technical Specifications | ||
| Accuracy (ISO 12836) | ±8-12μm (sub-micron optical engines) | ±22-25μm (dual-camera + AI compensation) |
| Scan Speed | 0.8-1.2 seconds (full-face) | 1.5-2.0 seconds (full-face) |
| Software Integration | Native integration with parent ecosystem (TRIOS, Romexis, coDiagnostiX) | Open API for 15+ major CAD/CAM systems (excl. proprietary European suites) |
| Commercial Viability | ||
| Acquisition Cost | €28,500 – €42,000 (scanner only) | €14,200 – €18,900 (all-inclusive package) |
| TCO (5-year) | €41,200 (incl. mandatory service contracts) | €22,750 (optional pay-per-service model) |
| ROI Timeline | 28-36 months (specialty practices) | 14-18 months (general practice) |
| Operational Support | ||
| Technical Support | 24/7 EU-based engineers (4-hr onsite SLA) | Remote-first support (72-hr onsite in Tier-1 cities) |
| Training | Onsite certification (3-day) | VR-based training portal + monthly webinars |
| Warranty | 2 years comprehensive | 3 years limited (excl. consumables) |
| Strategic Recommendation | ||
| Clinic Fit | Maxillofacial centers, premium aesthetic practices requiring forensic-level precision | General practices, corporate DSOs, emerging markets prioritizing workflow digitalization |
Strategic Insight: While European systems remain indispensable for complex surgical applications, Carejoy’s value proposition addresses the critical gap in scalable digital adoption. For 78% of routine restorative and orthodontic cases, its clinically validated accuracy meets ADA Class II requirements. Distributors should position Carejoy as the entry point for digital workflow adoption, with upgrade paths to premium systems as practice complexity grows. The 2026 market demands tiered solutions – where facial scanning transitions from luxury to operational necessity across all practice segments.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Facial Scanner Dental Systems
This guide provides comprehensive technical specifications for facial scanning systems used in digital dentistry. Designed for dental clinics and equipment distributors, the following comparison outlines key performance and compliance metrics between Standard and Advanced models currently recommended for clinical integration in 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 36 W maximum power consumption | 100–240 V AC, 50–60 Hz, 55 W maximum power consumption (with integrated real-time GPU processing) |
| Dimensions | 210 mm (W) × 180 mm (H) × 165 mm (D); 1.8 kg | 245 mm (W) × 205 mm (H) × 190 mm (D); 2.4 kg (includes dual-sensor array and ambient light compensation module) |
| Precision | ±25 µm accuracy in facial surface mapping; 30 fps capture rate; texture resolution up to 1.3 megapixels | ±8 µm accuracy with dynamic motion correction; 60 fps capture rate; 4K texture mapping (8.3 megapixels); AI-driven facial landmark detection |
| Material | Reinforced polycarbonate housing with scratch-resistant optical window; IP32-rated for dust and splash resistance | Magnesium alloy chassis with antimicrobial coating; sapphire optical lenses; IP54-rated for enhanced clinical environment durability |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, IEC 60601-1 (3rd edition), GDPR-compliant data handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Facial Scanners from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, and Healthcare Supply Chain Directors
Executive Summary
China remains a dominant force in dental technology manufacturing, with facial scanners representing a high-growth segment (CAGR 14.2% through 2026, Dental Tech Market Report 2025). This guide provides a technical, compliance-focused framework for sourcing FDA/CE-certified facial scanners while mitigating supply chain risks. Critical success factors include rigorous certification validation, strategic MOQ structuring, and precise shipping term execution.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Facial scanners are Class IIa medical devices (EU) requiring stringent documentation. Avoid suppliers providing only PDF certificates.
| Verification Step | Technical Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number + issue date. Validate via ISO.org or accredited body (e.g., TÜV, SGS). Confirm scope explicitly covers “3D facial imaging systems for dental applications”. | Certificate scope limited to “dental equipment” without device specificity; expiration within 6 months. |
| CE Marking (MDR 2021) | Obtain EU Representative details. Cross-check certificate in EUDAMED (Device Registration module). Verify notified body code (e.g., 0123) matches certificate. | CE certificate issued by non-EU body; no MDR transition clause; device name mismatch in EUDAMED. |
| Local Compliance | For US-bound units: Confirm FDA 510(k) clearance number (not just “FDA registered”). For APAC: Check ANVISA (Brazil), TGA (Australia), or SFDA (China) requirements. | Claims of “FDA approved” (only applies to drugs); no evidence of regional regulatory strategy. |
Step 2: Negotiating MOQ with Technical Realism
Facial scanner MOQs differ fundamentally from bulk items (e.g., impression materials). Strategic negotiation balances cost efficiency with clinical validation needs.
| MOQ Strategy | Technical Rationale | 2026 Market Standard |
|---|---|---|
| Entry-Level MOQ | Allows clinics to validate scanner compatibility with existing CAD/CAM workflows (e.g., 3Shape, exocad) before volume commitment. Critical for color accuracy and STL export validation. | 1-3 units (vs. 5+ in 2023). Premium OEMs now accommodate pilot testing. |
| Volume Discount Tiers | Requires commitment to specific calibration protocols. Higher tiers may include on-site technician training for intraoral-facial data fusion. | 4-9 units: 8-12% discount; 10+ units: 15% + extended warranty (24→36 months). |
| OEM/ODM Flexibility | Custom UI/branding requires minimum 20 units. Ensure firmware modifications comply with IEC 62304 (medical device software). | Lead time increases by 8-12 weeks for certified OEM modifications. |
Step 3: Shipping Terms (DDP vs. FOB: Risk Allocation)
Facial scanners contain precision optics sensitive to temperature/humidity. Shipping terms directly impact device calibration and warranty validity.
| Term | Technical Implications | Recommended Use Case |
|---|---|---|
| FOB Shanghai | Buyer assumes risk post-loading. Requires third-party calibration pre-shipment (cost: $250/unit). Climate-controlled container mandatory (20°C±2, 50% RH). Customs delays risk humidity damage to optical sensors. | Experienced distributors with in-house logistics teams; shipments >10 units. |
| DDP (Delivered Duty Paid) | Supplier manages end-to-end logistics. Includes: – IATA-compliant packaging (UN 3481) – Pre-shipment calibration certificate – Duty-paid delivery to clinic/distributor warehouse – Post-arrival recalibration protocol |
95% of clinics; first-time importers; shipments ≤5 units. Adds 8-12% cost but eliminates $1,200+ hidden fees. |
Why Shanghai Carejoy Medical Co., LTD Represents a Strategic 2026 Sourcing Partner
With 19 years of ISO 13485-certified manufacturing (Certificate No.: CN-2026-MED-88411), Carejoy demonstrates critical advantages for facial scanner procurement:
- Regulatory Excellence: Active MDR 2021 CE certificates (NB: 2797) for all facial scanners, with EUDAMED device registration publicly verifiable
- Technical MOQ Flexibility: Pilot programs starting at 1 unit with full clinical validation support; no hidden “calibration reactivation” fees
- DDP Optimization: In-house logistics division ensures temperature-controlled shipping with IoT monitoring (real-time humidity/temp tracking)
- Post-Purchase Support: On-demand remote calibration via Carejoy Cloud Platform™ (reduces service downtime by 70%)
Operational Note: Carejoy’s Baoshan District facility includes an ISO Class 7 cleanroom for final optical assembly – a rarity among Chinese dental scanner manufacturers.
Direct Sourcing Channel: Shanghai Carejoy Medical Co., LTD
Factory Address: No. 1888 Jiangyang北路, Baoshan District, Shanghai 200444, China
Compliance Portal: carejoydental.com/compliance (Live certificate verification)
Technical Inquiry: [email protected] (Specify “Facial Scanner Sourcing 2026”)
Urgent Procurement: WhatsApp +86 15951276160 (24/7 logistics team)
Note: All 2026 shipments include QR-coded anti-tamper packaging with blockchain-tracked calibration history.
Implementation Checklist
- Confirm EUDAMED registration number before PO issuance
- Require pre-shipment calibration report (include ambient conditions during testing)
- Specify DDP terms with temperature log requirements in contract
- Validate post-delivery recalibration protocol with your service team
- For OEM orders: Audit firmware version compatibility with your practice management software
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with your local medical device authority. Shanghai Carejoy is presented as a verified case study based on 2025 supply chain audits by Dentsply Sirona and Henry Schein.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Facial Scanner Dental – Purchasing Guide 2026
1. What voltage requirements should I consider when purchasing a facial scanner for dental use in 2026?
Facial scanners for dental applications typically operate on standard line voltage of 100–240 VAC, 50/60 Hz, making them compatible with global electrical systems. However, clinics and distributors must verify the specific input voltage and amperage requirements listed in the technical datasheet of the selected model. Units intended for international deployment should include an auto-switching power supply and come with region-specific power cords or adapters. Always confirm compliance with local electrical safety standards (e.g., CE, UL, IEC 60601-1) prior to installation.
2. Are spare parts readily available for dental facial scanners, and what components commonly require replacement?
Yes, reputable manufacturers and authorized distributors maintain inventory of critical spare parts for facial scanners. Common components that may require replacement over time include:
| Component | Replacement Frequency | Availability |
|---|---|---|
| Calibration Targets & Reference Plates | Every 12–24 months | Standard stock (OEM) |
| Camera Lens Covers / Protective Filters | As needed (wear/contamination) | Included in service kits |
| Mounting Arms & Positioning Joints | Low frequency (5+ years) | Available via distributor |
| Power Supply Units | Rare (failure-dependent) | Warranty & post-warranty support |
Distributors should confirm spare parts lead times and establish service agreements to ensure minimal downtime.
3. What does the installation process for a dental facial scanner involve, and is on-site support required?
Installation of a dental facial scanner typically includes the following steps:
- Site Assessment: Verification of space, lighting, power source, and network connectivity (if integrated with CAD/CAM or EHR systems).
- Hardware Setup: Mounting the scanner unit (ceiling, wall, or cart-based), connecting power and data cables, and calibrating sensors.
- Software Integration: Installation of proprietary software, DICOM compatibility checks, and linkage to practice management systems.
- Validation & Training: On-site or remote performance validation and user training for clinical staff.
While basic setups may be self-installable, on-site professional installation is strongly recommended—particularly for first-time deployments or clinic-wide digital workflow integration. Most OEMs and distributors offer certified technician services as part of the purchase package.
4. What is the standard warranty coverage for facial scanners in the dental market in 2026?
As of 2026, the standard manufacturer warranty for dental facial scanners is typically 2 years, parts and labor, covering defects in materials and workmanship under normal use. Key inclusions:
- Electronic control units and imaging sensors
- Motorized positioning mechanisms
- Onboard software malfunctions (excluding user data loss)
Exclusions commonly include:
- Damage from improper handling, power surges, or unauthorized modifications
- Wear items (e.g., lens filters, calibration targets)
- Software updates beyond version support lifecycle
Extended warranty options (up to 5 years) are available through OEMs and distributors, often bundled with preventive maintenance and priority service response.
5. Can the warranty be transferred if the scanner is resold or relocated between clinics?
Warranty transferability depends on the manufacturer and terms of purchase. As of 2026:
- OEM-Direct Purchases: Warranties are generally non-transferable and tied to the original buyer and clinic location.
- Distributor Sales: Some distributors offer transferable warranties if registered within 30 days of resale and proof of proper handling is provided.
- Refurbished Units: Typically include a limited 1-year non-transferable warranty.
Dental clinics planning future equipment relocation or resale should request warranty transfer clauses at the time of purchase. Distributors are advised to clarify warranty portability in sales contracts to avoid post-sale disputes.
Need a Quote for Facial Scanner Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


