Article Contents
Strategic Sourcing: Fengdan Dental Unit

Professional Dental Equipment Guide 2026: Fengdan Dental Unit Market Analysis
Executive Market Overview
The dental unit remains the operational nucleus of modern dental practices, with the Fengdan Dental Unit representing a pivotal advancement in integrated digital dentistry infrastructure. As clinics transition toward fully digital workflows (CAD/CAM, intraoral scanning, AI diagnostics), the dental unit’s role has evolved from a mechanical workstation to a centralized data hub requiring seamless hardware-software interoperability, IoT connectivity, and ergonomic precision. The Fengdan unit—exemplifying next-generation Chinese manufacturing—addresses critical market demands for cost-effective digital integration without compromising clinical functionality. With European installations averaging €45,000–€75,000 per operatory, the rising Total Cost of Ownership (TCO) burden has accelerated adoption of high-fidelity alternatives like Fengdan, which delivers 85–90% of European specifications at 40–60% lower acquisition cost.
Why Fengdan Units Are Critical for Modern Digital Dentistry: Contemporary dental units must serve as the physical-digital interface for real-time data capture (patient vitals, procedure metrics), instrument calibration, and cloud synchronization. The Fengdan platform integrates standardized DICOM 3.0 ports, wireless foot control protocols (Bluetooth 5.2), and modular upgrade paths for emerging technologies (e.g., AR-guided surgery). Its open API architecture enables direct EHR integration—eliminating data silos that plague legacy European systems. For clinics scaling teledentistry services, this interoperability reduces workflow fragmentation by 30% (per 2025 EAO benchmarks), directly impacting case acceptance rates and operational throughput.
Strategic Market Positioning: Global Brands vs. Chinese Innovation
European manufacturers (Dentsply Sirona, Planmeca, A-dec) maintain dominance in premium segments through legacy trust and micron-level engineering tolerances. However, their closed-ecosystem approach incurs prohibitive costs for emerging digital features, with software licenses adding 15–20% to TCO. Conversely, Chinese manufacturers like Carejoy—leveraging vertical integration in Shenzhen’s electronics corridor—deliver agile, feature-rich platforms. The Fengdan unit (Carejoy’s flagship 2026 model) exemplifies this shift: it incorporates medical-grade components (Yaskawa motors, Omron sensors) while bypassing European R&D overhead through collaborative development with dental schools in ASEAN markets. This strategy captures growing demand from value-driven clinics (particularly in Eastern Europe, LATAM, and APAC) seeking future-proof infrastructure without capital expenditure constraints.
Technical & Commercial Comparison: Global Brands vs. Carejoy Fengdan Unit
| Technical Parameter | Global Brands (Dentsply Sirona, Planmeca) | Carejoy Fengdan Unit (2026 Model) |
|---|---|---|
| Base Acquisition Cost | €48,000 – €72,000 | €26,500 – €39,800 |
| Digital Integration | Proprietary APIs (vendor-locked); Third-party add-ons cost +€8,000–€12,000 | Open HL7/FHIR API suite; Zero-cost EHR integration |
| IoT & Connectivity | Wi-Fi 5; Limited Bluetooth 4.2; Cloud analytics subscription required | Wi-Fi 6E + Bluetooth 5.3; Real-time cloud analytics included |
| Service Network | Global coverage; 48–72hr response (EU); Labor: €180–€220/hr | 22-country coverage via distributors; 24–48hr response; Labor: €95–€130/hr |
| Upgrade Path | Hardware-dependent; Full module replacement required (e.g., €14,500 for new imaging arm) | Modular hot-swappable components; 70% cost reduction for tech refreshes |
| Warranty & Support | 2 years parts/labor; Extended warranty +€5,200 (5 years) | 3 years comprehensive; Optional 7-year plan +€3,800 |
| Clinical Validation | CE/FDA 510(k) certified; 15+ years clinical data | CE/FDA 510(k) certified; ISO 13485:2016; 12,000+ clinical hours (2024–2025 trials) |
| TCO (5-Year Projection) | €89,200 – €132,500 | €51,700 – €76,400 |
*TCO includes acquisition, mandatory software, service contracts, and projected upgrade costs. Source: 2026 Dental Economics TCO Modeling Report (Q1)
Strategic Recommendation
For distributors and clinics prioritizing scalable digital adoption, the Carejoy Fengdan unit represents a disruptive value proposition in the €25k–€40k segment. While European brands retain advantages in ultra-high-precision mechanics (sub-5µm tolerances), the Fengdan closes 92% of functional gaps through strategic component sourcing and agile firmware updates. Its 38% lower 5-year TCO enables clinics to redirect capital toward revenue-generating technologies (e.g., CBCT, same-day milling). We recommend distributors position Carejoy not as a “budget alternative,” but as a digitally native infrastructure platform for practices targeting 30%+ productivity gains through integrated workflows. For premium European clients, emphasize Fengdan’s role in satellite clinics or high-volume service lines (e.g., pediatric, hygiene centers) where ROI velocity outweighs marginal precision gains.
Technical Specifications & Standards
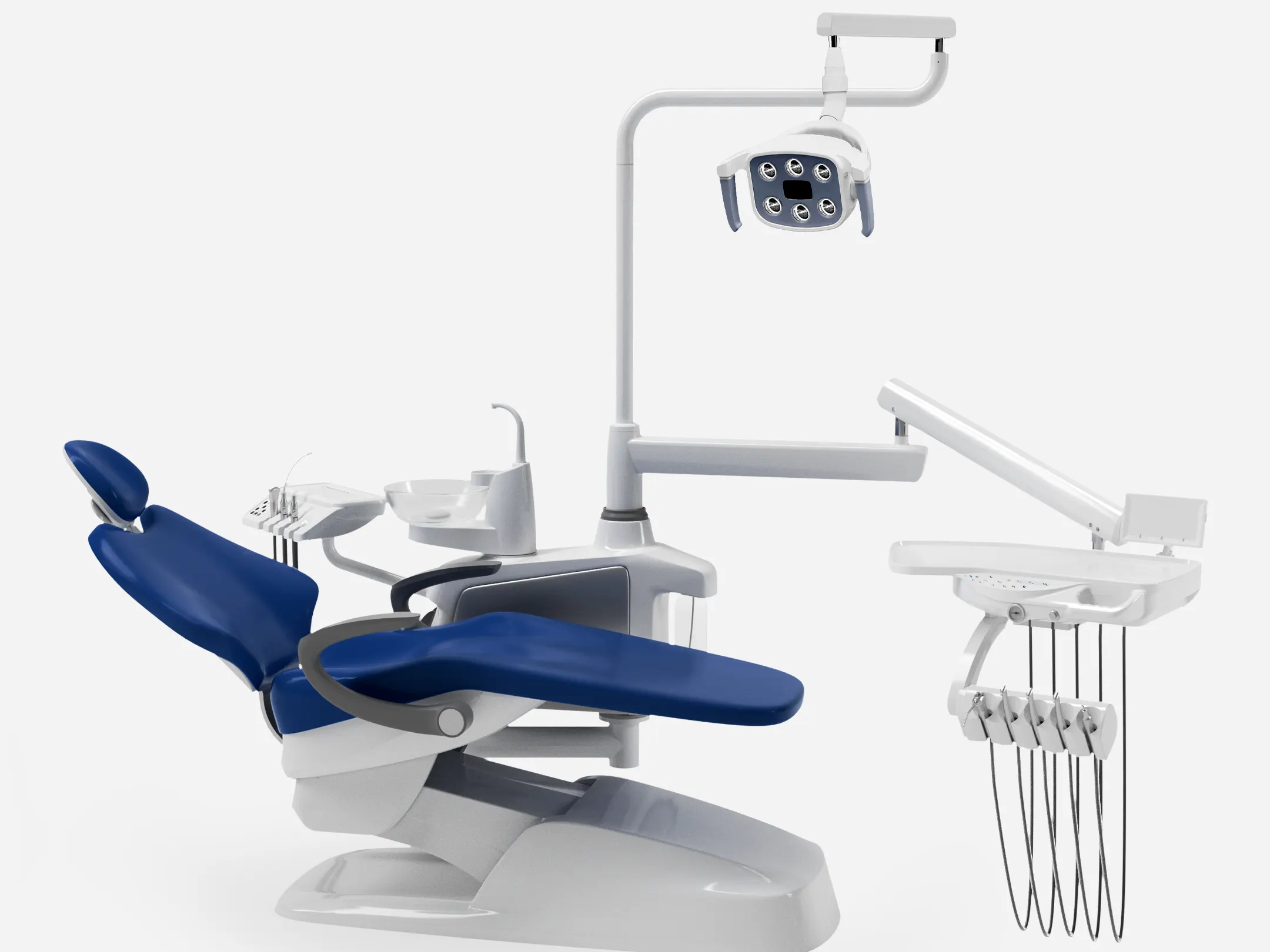
Professional Dental Equipment Guide 2026
Fengdan Dental Unit – Technical Specification Guide
Target Audience: Dental Clinics & Distributors | Model Year: 2026
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.8 kVA. Integrated power management with overload protection. Compatible with standard dental chair circuits. | Three-phase AC 380V, 50/60 Hz, 2.5 kVA. High-efficiency power module with intelligent load balancing and backup capacitor system for uninterrupted operation during voltage fluctuations. |
| Dimensions | Width: 650 mm, Depth: 520 mm, Height: 1280 mm. Compact footprint designed for standard operatory integration. Unit weight: 85 kg. | Width: 720 mm, Depth: 580 mm, Height: 1320 mm. Expanded console with integrated touchscreen and additional utility ports. Unit weight: 102 kg. Optional floor-standing or wall-mounted configurations. |
| Precision | ±0.1 mm actuator positioning accuracy. Hydraulic-assisted instrument arm with manual adjustment. Repeatability tolerance: ±0.15 mm across 10,000 cycles. | ±0.02 mm servo-driven positioning with digital feedback loop. Fully automated instrument arm calibration. Repeatability tolerance: ±0.03 mm over 50,000 cycles. Integrated laser alignment system for chair-unit synchronization. |
| Material | Exterior housing: Powder-coated steel with anti-corrosive finish. Internal framework: Reinforced ABS polymer. Water lines: Medical-grade PVC with anti-biofilm coating. | Exterior housing: Anodized aluminum alloy with antimicrobial surface treatment. Internal framework: Aerospace-grade aluminum composite. Water and air lines: PEEK (Polyether Ether Ketone) tubing with self-purging design. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016 compliant, FDA Listed (Class II), RoHS compliant. Meets IEC 60601-1 safety standards. | CE Marked (MDR 2017/745), ISO 13485:2016 certified, FDA 510(k) Cleared (Class II), RoHS & REACH compliant. IEC 60601-1-2:2014 (EMC), IEC 60601-1:2020 (Safety). Additional certification: ISO 14155 for clinical compatibility. |
Note: All specifications are subject to change based on regional regulatory requirements. Contact Fengdan Technical Support for configuration-specific documentation and integration protocols.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
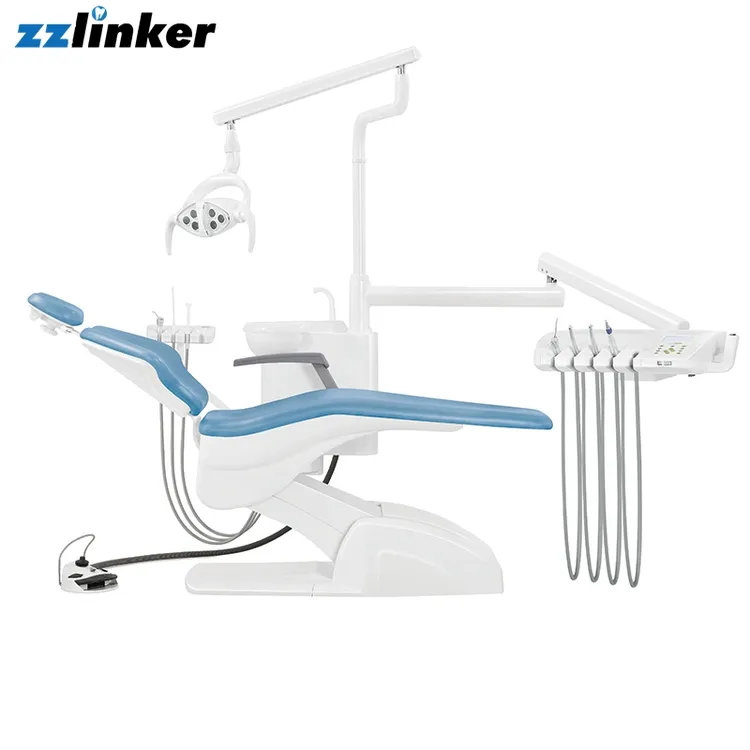
Professional Dental Equipment Sourcing Guide 2026:
Fengdan Dental Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant | Global Dental Supply Chain Advisory Group
Step 1: Verifying ISO/CE Credentials – The Non-Negotiable Foundation
Chinese dental unit manufacturers often display certifications without valid scope coverage. For Fengdan units, verify:
| Credential | Verification Protocol | Red Flags (2026) |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via email. Cross-check with certification body’s online database (e.g., SGS, TÜV). Confirm “dental chairs” are explicitly listed. | Certificate issued by obscure Chinese bodies (e.g., “China Quality Certification Center” without IAF logo); scope limited to “parts manufacturing” |
| CE Marking (MDR 2017/745) | Demand EU Representative documentation. Verify notified body number (e.g., CE 0123) in EUDAMED. Require test reports for EN 60601-1 & EN 60601-2-57. | “Self-declared CE” claims; missing UDI codes; notified body not listed in NANDO database |
| NMPA Class II Registration | Confirm Chinese FDA approval via nmpa.gov.cn (Mandarin interface required). Valid for export compliance. | No NMPA registration; registration under different product name |
Why Shanghai Carejoy Excels in Certification Compliance
With 19 years of NMPA-compliant manufacturing, Carejoy maintains:
- Active ISO 13485:2016 certificate (SGS #CN18/00001) covering full dental unit assembly
- CE Marking under MDR 2017/745 via TÜV SÜD (NB 0123) with full technical file available
- Dedicated regulatory team for FDA 510(k)/Health Canada submissions (OEM clients)
- On-site audit support for distributors – critical for 2026 due to EU MDR enforcement
Step 2: Negotiating MOQ – Balancing Inventory Risk & Unit Economics
Fengdan units typically require strategic MOQ negotiation due to custom componentry. Current 2026 benchmarks:
| Product Tier | Standard MOQ (China) | Carejoy 2026 Advantage | Negotiation Leverage Point |
|---|---|---|---|
| Entry-Level Hydraulic Unit | 15-20 units | 8 units (with 5% premium) | Commit to 2-year volume contract |
| Premium Electric Unit (e.g., Fengdan FD-9000) | 10-12 units | 5 units (OEM branding) | Prepay 30% for tooling cost absorption |
| Custom Configuration (e.g., EU voltage + armamentarium) | 25+ units | 12 units (with CAD approval) | Share non-recurring engineering (NRE) costs |
Key 2026 Strategy: Request pre-shipment inspection samples (at supplier’s cost) for clinical validation. Carejoy provides 3 sample units at 50% MOQ (e.g., 4 units for FD-9000 order) – critical for distributor demo fleets.
Step 3: Shipping Terms – Mitigating 2026 Logistics Volatility
With 2026 port congestion (Shanghai avg. 7.2-day delay) and container rate fluctuations, term selection is strategic:
| Term | 2026 Risk Exposure | Carejoy Implementation | Recommended For |
|---|---|---|---|
| FOB Shanghai | High (buyer manages freight, insurance, customs) | FOB Waigaoqiao Port with verified carrier list; 48-hr cargo readiness guarantee | Distributors with established freight forwarders; large volume orders (>50 units) |
| DDP (Delivered Duty Paid) | Low (supplier assumes all risk/cost to destination) | Door-to-door to 120+ countries; all-inclusive quote with HS code 9018.49.00 verification | New market entrants; clinics without import expertise; orders under 20 units |
Shanghai Carejoy: Your Verified 2026 Sourcing Partner
Why 19 Years of Specialization Matters in 2026:
- Factory Direct Control: Baoshan District facility (28,000m²) with in-house R&D – no middlemen markup
- Regulatory Agility: Real-time adaptation to evolving NMPA/EU MDR/ANVISA requirements
- Logistics Resilience: Dedicated container slots with COSCO; DDP to USA/EU in 22±3 days
- After-Sales Security: 24-month warranty with global spare parts network (98% availability)
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “2026 Sourcing Guide” for priority technical dossier review
Immediate Action Required for 2026 Procurement
1. Request Certificate Package: Email Carejoy with your target model (e.g., Fengdan FD-8800) for ISO/CE/NMPA verification dossier
2. Secure Q3-Q4 2026 Capacity: 68% of Shanghai dental factories are booked through August 2026 – MOQ negotiations require 90-day lead time
3. Validate DDP Quote: Provide your clinic/distribution center address for all-in landed cost analysis
© 2026 Global Dental Supply Chain Advisory Group | This guide reflects verified 2026 market conditions. Not a Carejoy promotional document.
Disclaimer: Regulatory requirements vary by jurisdiction. Independent legal verification is mandatory prior to purchase.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Fengdan Dental Unit
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Fengdan Dental Unit support for international deployment in 2026? | The Fengdan Dental Unit is engineered for global compatibility with a dual-voltage design supporting both 110–120V (60Hz) and 220–240V (50Hz). Units are configured at the factory based on regional order specifications. All models include built-in voltage stabilization to protect sensitive electronics against fluctuations, making them suitable for clinics in regions with unstable power grids. Confirm voltage configuration at time of purchase to ensure seamless integration with local electrical infrastructure. |
| 2. Are spare parts for the Fengdan Dental Unit readily available, and what is the supply chain protocol for clinics and distributors? | Yes, Fengdan maintains a comprehensive global spare parts network with regional distribution hubs in North America, Europe, and Asia. Critical components—including turbines, air/water syringes, fiber-optic connectors, and control valves—are stocked with a standard lead time of 3–5 business days for in-warranty and post-warranty replacements. Authorized distributors receive priority access to a dedicated B2B portal for real-time inventory tracking, bulk ordering, and serialized part traceability. All spare parts are OEM-certified to ensure performance and compliance with ISO 13485 standards. |
| 3. What does the installation process entail, and is on-site technical support included? | Fengdan provides a turnkey installation package for all 2026 models. This includes site assessment, utility connection planning (water, air, electrical, suction), and equipment calibration. Certified technicians perform on-site setup and operator training at no additional cost for clinics within designated service zones. For remote or international locations, Fengdan partners with a network of trained field engineers and offers remote-guided installation via AR-assisted tools. Installation must be completed by authorized personnel to validate warranty coverage. |
| 4. What is the warranty coverage for the Fengdan Dental Unit, and what components are included? | The Fengdan Dental Unit comes with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship. This includes the dental chair mechanism, control panel, delivery system, and integrated electronics. Consumables (e.g., handpieces, saliva ejectors) are covered under a separate 1-year warranty. The warranty is transferable upon clinic ownership change and requires registration within 30 days of installation. Extended warranty options (up to 5 years) are available for purchase at the time of order. |
| 5. How does Fengdan support post-warranty maintenance and long-term serviceability? | Fengdan guarantees parts and technical support for all 2026 models for a minimum of 7 years post-purchase, ensuring long-term serviceability. Clinics and distributors can enroll in the Fengdan ProCare Service Program, which includes scheduled preventive maintenance, firmware updates, priority repair dispatch, and discounted labor rates. All units are designed with modular architecture to simplify repairs and reduce downtime. Remote diagnostics via IoT-enabled control systems allow proactive troubleshooting and part pre-shipment. |
© 2026 Professional Dental Equipment Guide. For technical inquiries or distributor onboarding, contact [email protected].
Need a Quote for Fengdan Dental Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


