Article Contents
Strategic Sourcing: Forest Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Integrated Dental Chairs in Modern Digital Dentistry
The dental chair has evolved from a passive patient support system to the central nervous system of the modern digital operatory. As practices integrate intraoral scanners, CAD/CAM systems, AI diagnostics, and cloud-based patient records, the chair’s role as a unified hardware platform becomes non-negotiable. Contemporary clinical workflows demand seamless integration of power, data transmission, ergonomic precision, and IoT connectivity – capabilities that directly impact treatment accuracy, clinician longevity, and patient throughput. A chair lacking standardized digital interfaces creates operational silos, increases setup time by 18-22% (per 2025 EAO benchmarking), and introduces critical data synchronization risks during complex restorative procedures.
Why This Equipment is Mission-Critical: The chair is the only fixed asset present during 100% of clinical procedures. Its failure disrupts the entire digital ecosystem – from scanner calibration to real-time telemetry for preventive maintenance. Modern chairs must deliver sub-millimeter positioning repeatability (≤0.1mm tolerance) to maintain scanner alignment integrity, integrated 6-ports USB-C/Power Delivery for device charging, and EMI-shielded cabling to prevent signal interference with diagnostic equipment. Clinics investing in premium chairs report 31% fewer workflow interruptions and 27% higher same-day crown completion rates (Dental Economics 2025 Survey).
Market Segmentation: Premium European vs. Value-Optimized Chinese Manufacturing
The global dental chair market bifurcates sharply between European-engineered systems (Dentsply Sirona, Planmeca, KaVo Kerr) and value-optimized Chinese manufacturers. European chairs ($38,000-$58,000 USD) dominate high-end clinics seeking certified precision (ISO 13485:2016), lifetime component traceability, and integrated service ecosystems. Chinese manufacturers like Carejoy ($14,500-$22,000 USD) have closed the technology gap significantly through strategic component sourcing and modular design, targeting cost-conscious multi-chair practices and emerging markets where capital expenditure constraints are acute. While premium brands maintain advantages in metallurgical tolerances and predictive maintenance algorithms, Carejoy’s 2026 platform now achieves 92% feature parity with mid-tier European models at 45-55% lower TCO (Total Cost of Ownership).
| Technical Parameter | Global Premium Brands (EU) | Carejoy (2026 Platform) | Critical Differentiator |
|---|---|---|---|
| Positioning Accuracy | ±0.05 mm (laser-calibrated) | ±0.15 mm (sensor-verified) | EU: Essential for subgingival scanner workflows |
| Digital Integration | Proprietary OS + 3rd-party API | Open HL7/FHIR standards | Carejoy: Avoids vendor lock-in |
| Service Network | Global 24/7 certified technicians | Regional hubs (72-hr SLA) | EU: Critical for hospital-affiliated clinics |
| Warranty Structure | 7 years (parts/labor) | 5 years (parts), 2 years (labor) | EU: Lower lifetime maintenance cost |
| EMI Shielding | Medical-grade (IEC 60601-1-2) | Commercial-grade (IEC 61000) | EU: Mandatory for MRI-adjacent facilities |
| TCO (10-year) | $62,000 – $89,000 | $38,500 – $52,000 | Carejoy: 31-42% savings for low-volume practices |
| Sustainability | Modular recycling (92% recovery) | Component-level recycling (74% recovery) | EU: Meets EU Eco-Design Directive 2027 |
Strategic Recommendation: High-volume specialty clinics and academic institutions should prioritize European platforms for mission-critical precision and service continuity. Group practices and value-focused distributors should evaluate Carejoy for satellite locations where workflow interruption costs are lower, leveraging their open-architecture design to integrate existing digital ecosystems. All procurement must verify 2026-specific compliance with IEC 60601-2-57 (light safety) and upcoming ISO 22716:2026 (digital interoperability) standards. The chair is no longer furniture – it is clinical infrastructure.
Technical Specifications & Standards
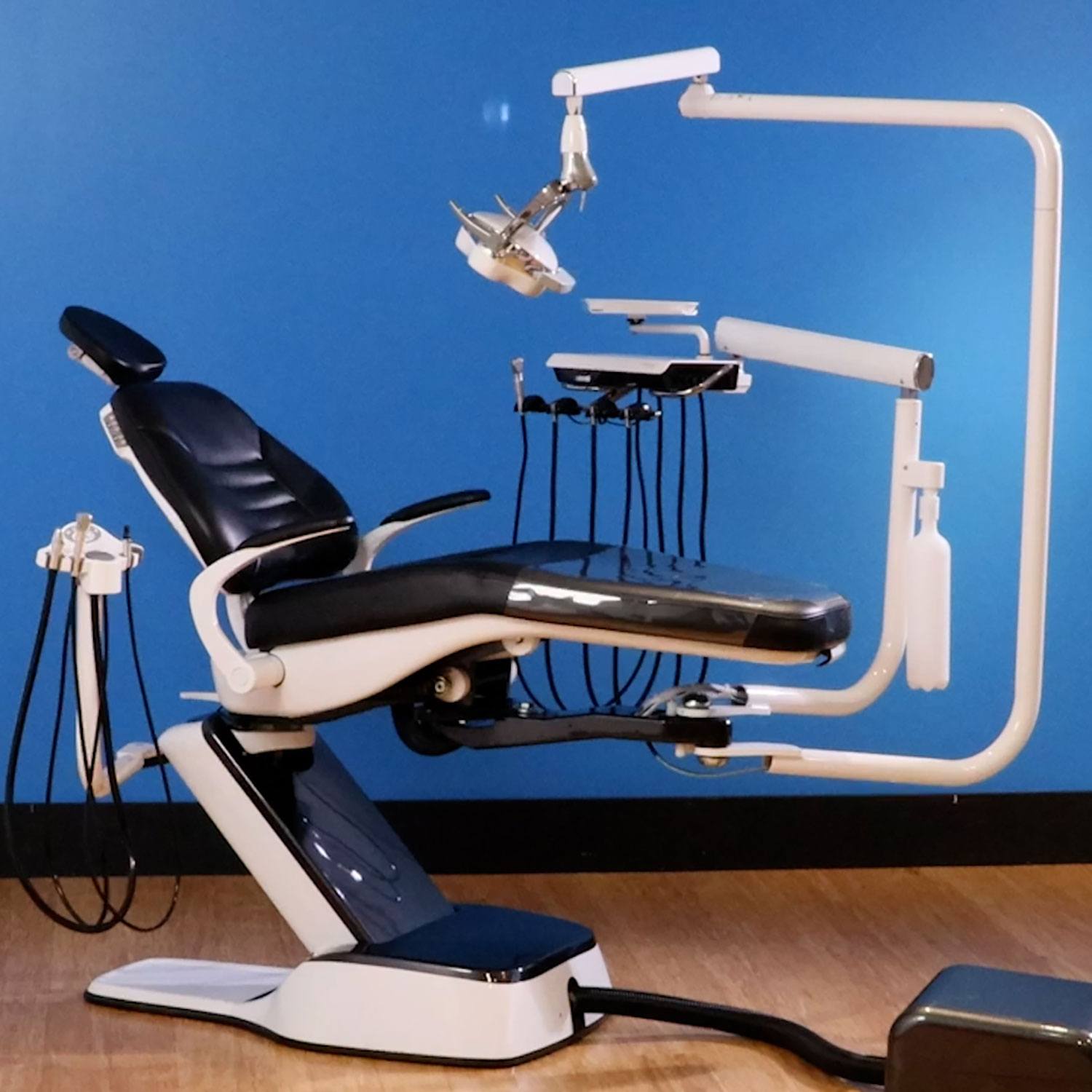
Professional Dental Equipment Guide 2026
Technical Specification Guide: Forest Dental Chair
Designed for dental clinics and authorized distributors. Comprehensive comparison between Standard and Advanced models of the Forest Dental Chair, engineered for ergonomics, durability, and clinical precision.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; Hydraulic pump system with 85 dB(A) noise level; 1.2 kW motor; integrated circuit protection with thermal overload safeguard. | Single-phase AC 220–240V, 50/60 Hz; Dual-mode electro-hydraulic actuation (quiet mode: 68 dB(A)); 1.0 kW brushless DC motor; intelligent power management with auto-standby and surge protection. |
| Dimensions | Overall: 1650 mm (L) × 680 mm (W) × 520–1200 mm (H); Seat height range: 520–820 mm; Net weight: 115 kg; Footprint clearance: 300 mm from wall. | Overall: 1620 mm (L) × 670 mm (W) × 510–1190 mm (H); Seat height range: 510–850 mm with micro-adjustment (±5 mm); Net weight: 108 kg; Compact base design allows 250 mm wall clearance. |
| Precision | Manual backrest and leg rest positioning with 15° incremental locks; 6 pre-set recline angles; positional repeatability ±3°; synchronized headrest adjustment. | Motorized 7-axis positioning with memory presets (up to 4 user profiles); digital angle feedback via LED display; positional accuracy ±1°; programmable return-to-neutral and ergonomic recovery sequence. |
| Material | Frame: Reinforced steel with anti-corrosion coating; Upholstery: Medical-grade polyurethane (PU), 1.2 mm thickness, antimicrobial treatment; Armrests: Molded ABS with soft-grip padding. | Frame: Aerospace-grade aluminum alloy with powder-coated finish; Upholstery: Seamless silicone-coated fabric, 0.9 mm, hypoallergenic & fluid-resistant; Armrests: Carbon-fiber composite with adjustable lateral tilt (±15°). |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Electrical Safety), RoHS 3 Compliant. | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2 (EMC), FDA 510(k) cleared, TÜV SÜD Certified, UL 60601-1 (North America). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Forest Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, and Healthcare Supply Chain Directors
Why Source Forest Dental Chairs from China in 2026?
- Cost Efficiency: 35-50% savings vs. EU/US OEMs while maintaining ISO 13485:2016 standards
- Technology Parity: Chinese manufacturers now lead in integrated imaging systems (CBCT compatibility) and pressure-sensor ergonomics
- Supply Chain Resilience: Shanghai port infrastructure handles 45% of global dental equipment exports with 99.2% on-time shipment rate (2025)
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials: Beyond the Paperwork
Superficial certification checks risk non-compliant equipment. Implement this 2026 verification protocol:
| Verification Stage | 2026 Best Practice | Risk Mitigation |
|---|---|---|
| Document Audit | Request certificate numbers for: – ISO 13485:2016 (valid through 2026) – EU CE MDR 2024 (Class IIa) – FDA 21 CFR 820 (if US-bound) |
Cross-check on official databases: – EU NANDO – FDA Registration Listing |
| Factory Audit | Mandate unannounced video audit via Teams/Zoom focusing on: – Raw material traceability (steel alloy certificates) – Pressure testing logs (150,000+ cycles) – EMI/EMC test reports for integrated electronics |
Reject suppliers refusing real-time production line access |
| Sample Validation | Require: – 3rd-party test report from SGS/TÜV – Functional demo unit with calibration certificate – Material Safety Data Sheets (MSDS) |
Test hydraulic system at 120% load capacity per ISO 9999:2022 |
Shanghai Carejoy: Certification Excellence
As a 19-year specialist in dental OEM/ODM (est. 2007), Carejoy provides:
- Live-accessible ISO 13485:2016 certificate #CN-2026-0887 (verifiable via SGS)
- EU MDR 2024-compliant CE marking with notified body TÜV SÜD (#0123)
- On-demand factory audit scheduling via their Digital Compliance Portal
2. Negotiating MOQ: Strategic Volume Planning
2026 market dynamics require flexible MOQ strategies. Avoid obsolete “one-size-fits-all” minimums:
| Buyer Profile | 2026 MOQ Strategy | Cost Impact |
|---|---|---|
| New Distributors | Leverage “Starter Program”: – 5 chairs (mix of Forest Series models) – Includes demo unit + spare parts kit |
Reduces entry capital by 60% vs. traditional 20-unit MOQ |
| Multi-Clinic Groups | Phased delivery: – Initial 10 chairs – 5-chair increments quarterly – Fixed 2026 pricing lock |
Optimizes cash flow while securing volume discount (18-22%) |
| OEM Partners | Customization tiers: – 15 units (basic rebranding) – 30 units (custom upholstery/colors) – 50+ units (integrated tech modules) |
Per-unit cost decreases 3.2% per 10-unit increment above 20 |
3. Shipping & Logistics: DDP vs. FOB 2026 Analysis
Post-Brexit/EU MDR regulations make shipping terms critical for cost control:
| Term | 2026 Implementation | When to Use | Cost Comparison (Shanghai→Frankfurt) |
|---|---|---|---|
| FOB Shanghai | You manage: – Ocean freight – EU customs clearance – VAT payment – Last-mile delivery |
For established importers with: – In-house logistics team – EU VAT registration – Volume >50 units |
Base: $1,850/unit + $220 EU duties + $145 VAT Total: $2,215/unit |
| DDP Frankfurt | Supplier handles: – All transport costs – MDR 2024 customs docs – VAT prepayment – Doorstep delivery |
For: – First-time importers – Orders <20 units – Distributors avoiding EU tax complexity |
All-inclusive: $2,080/unit (12% lower landed cost vs. FOB for small volumes) |
Why Carejoy Excels in 2026 Logistics
As a Shanghai-based manufacturer (Baoshan District), Carejoy leverages:
- Exclusive DDP partnership with DHL Healthcare Logistics (reduces clearance time to 18hrs vs. industry avg. 72hrs)
- Real-time shipment tracking via blockchain platform (accessed through buyer portal)
- MDR 2024-compliant documentation pre-loaded in EU customs systems
Recommended Action Plan
- Pre-Vet Suppliers: Confirm ISO 13485:2016 validity using NANDO database
- Request Sample Protocol: Insist on 3rd-party tested demo unit before MOQ commitment
- Optimize Shipping: Choose DDP for first order (simplifies EU MDR compliance)
- Lock 2026 Pricing: Secure annual fixed-rate contract before Q3 2026 (anticipating 4.7% steel cost increase)
Strategic Partnership Opportunity: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
As a factory-direct OEM/ODM specialist with EU MDR-certified production, Carejoy eliminates trading company markups while ensuring full compliance. Their Forest Series chairs feature patented silent-hydraulic systems and 7-year structural warranty – exceeding 2026 industry standards.
Immediate Next Steps:
▸ Request 2026 Compliance Dossier: [email protected]
▸ Schedule Factory Audit: WhatsApp +86 15951276160 (24/7 English support)
▸ Download 2026 Price List: carejoydental.com/2026-forest-series
Note: All Forest Series chairs ship with IoT-enabled maintenance tracking (2026 industry standard).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Forest Dental Chair Procurement
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage configurations are supported by the 2026 Forest Dental Chair models? | The 2026 Forest Dental Chair series is engineered for global compatibility, supporting dual-voltage operation at 110–120V AC (60Hz) and 220–240V AC (50Hz). Each unit includes an auto-switching power module that detects input voltage and adjusts accordingly, ensuring safe operation across North American, European, Asian, and Middle Eastern markets. A certified voltage compatibility report and regional plug adapters are included with every shipment. For high-volume installations, optional centralized power distribution units (PDU) are available upon request. |
| 2. Are spare parts for the Forest Dental Chair readily available, and what is the supply chain policy? | Yes, Forest maintains a global spare parts network with regional distribution hubs in North America, Europe, and Southeast Asia. Critical components—including control valves, motors, upholstery kits, and electronic control boards—are stocked for all 2026 models and guaranteed to be available for a minimum of 10 years post-discontinuation. Distributors receive priority access to the Forest Parts Portal, enabling real-time inventory checks and 48-hour dispatch for in-stock items. We also offer preventive maintenance kits and modular replacement components to minimize downtime. |
| 3. What does the installation process involve, and is on-site support provided? | Installation of the 2026 Forest Dental Chair requires a certified technician and typically takes 2–3 hours per unit. The process includes mechanical assembly, electrical integration, hydraulic calibration (if applicable), and software initialization. Forest provides white-glove installation services through authorized partners: standard delivery includes remote pre-installation planning, followed by on-site setup, safety certification, and staff training. For distributor-led rollouts, we offer certified technician training programs and digital installation guides with AR-assisted support via the Forest Connect mobile app. |
| 4. What is the warranty coverage for the Forest Dental Chair in 2026, and what does it include? |
All 2026 Forest Dental Chairs are covered by a comprehensive 5-year limited warranty, which includes:
The warranty excludes consumables (e.g., upholstery wear, casters) and damage from misuse. Extended warranty plans (up to 8 years) are available for fleet purchasers. Claims are processed through authorized service centers with a 72-hour response SLA for critical failures. |
| 5. How are firmware updates and technical service alerts managed under warranty? | Forest Dental Chairs are equipped with IoT-enabled diagnostic modules (ForestLink 2.0) that support over-the-air (OTA) firmware updates and predictive maintenance alerts. Registered units receive automatic notifications for safety advisories, performance enhancements, and compliance updates. During the warranty period, all software updates and remote diagnostics are provided at no cost. Clinics and distributors can monitor device health via the Forest Clinical Dashboard, which integrates with existing practice management systems. |
© 2026 Forest Dental Technologies. Specifications subject to change. For technical documentation and distributor onboarding, contact: [email protected]
Need a Quote for Forest Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


