Article Contents
Strategic Sourcing: Formlabs Dental 3D Printer

Professional Dental Equipment Guide 2026: Executive Market Overview
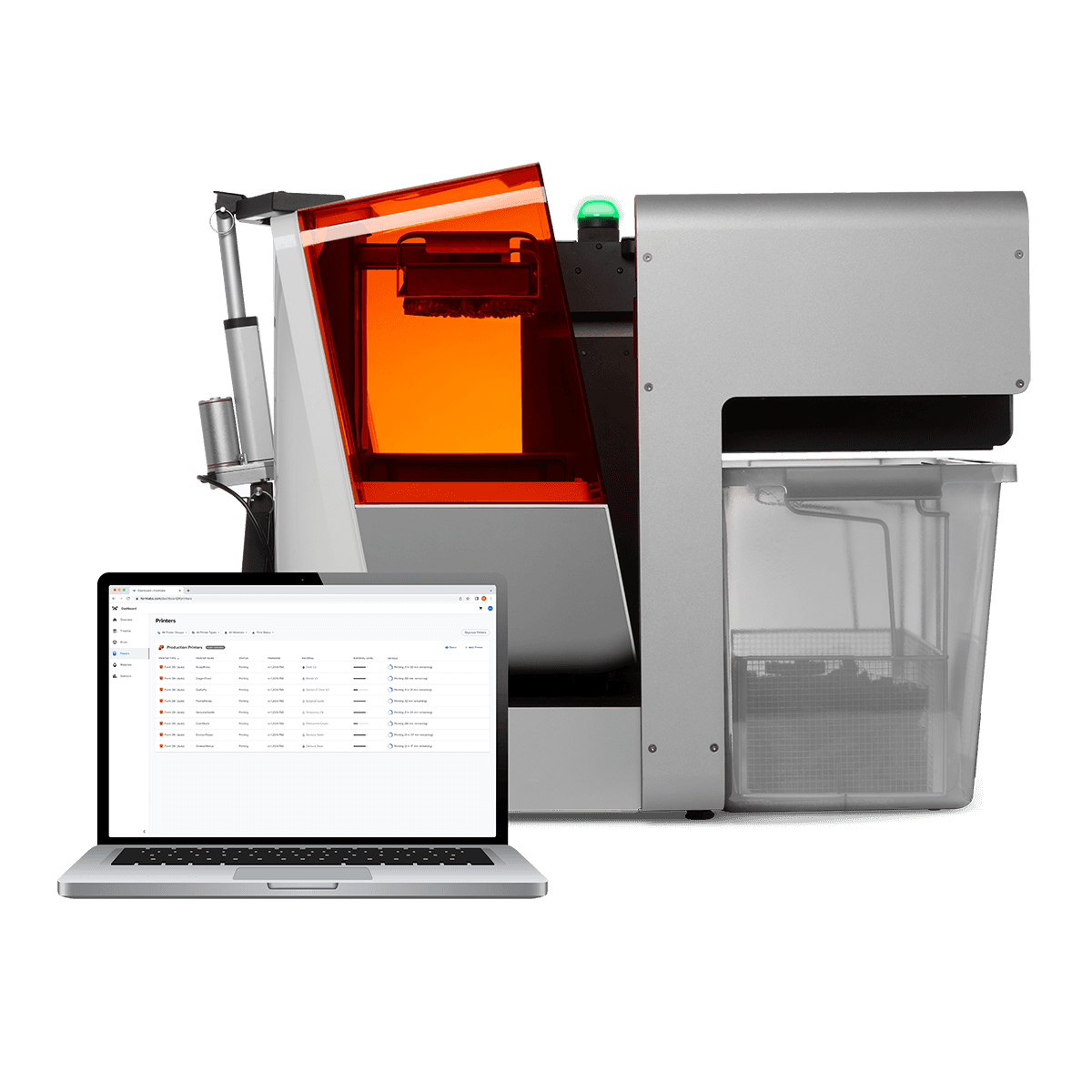
Formlabs Dental 3D Printing Systems – The Strategic Imperative for Modern Digital Workflows
The global dental 3D printing market is projected to exceed $4.8B by 2026 (CAGR 18.2%), driven by the irreversible shift toward digital dentistry. Central to this transformation are high-precision, accessible industrial-grade printers like the Formlabs Form 4D Dental and Form 4B Dental. These systems have evolved from niche tools to core clinical infrastructure, enabling same-day crown fabrication, surgical guide production, orthodontic models, and denture frameworks with laboratory-grade accuracy. For clinics, this translates to 30-50% reduction in third-party lab costs, 70% faster turnaround times for restorations, and enhanced patient retention through immediate treatment delivery. Distributors must recognize that integration of such platforms is no longer optional—it is the foundation of competitive, future-proof practice economics.
Strategic Value Proposition for Dental Practices
Formlabs systems address critical pain points in the digital workflow:
- Material Versatility: Certified biocompatible resins (e.g., Dental LT Clear, Surgical Guide Resin) covering 95% of restorative/orthodontic applications
- Laboratory-Grade Precision: ±25μm accuracy meets ISO 12836 standards for definitive prosthetics
- Seamless Ecosystem Integration: Native compatibility with major CAD platforms (exocad, 3Shape) via PreForm software
- Operational ROI: Payback period under 14 months for high-volume clinics (based on 15+ units/month production)
Failure to adopt such technology risks marginalization as 68% of EU patients now expect same-day restorations (2025 EAO Survey).
Market Segmentation: Premium European Brands vs. Value-Driven Chinese Manufacturers
The competitive landscape bifurcates sharply between established European engineering (high capital cost, premium service) and Chinese innovators (aggressive pricing, evolving quality). While German/Dutch brands (e.g., EnvisionTEC, 3D Systems) dominate high-end lab segments, Chinese manufacturers like Carejoy are rapidly capturing budget-conscious clinics and emerging markets through cost disruption. Formlabs occupies the strategic middle ground—delivering 90% of European precision at 40% lower TCO than legacy European systems.
Comparative Analysis: Global Premium Brands vs. Carejoy Dental 3D Printers
| Parameter | Global Premium Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Entry Price Range (2026) | €58,000 – €82,000 | €18,500 – €24,000 |
| XY Axis Accuracy | ±15-20μm | ±35-45μm |
| Material Compatibility | Full biocompatible resin ecosystem (ISO 10993 certified); 12+ dental-specific materials | Limited biocompatible options (5-7 materials); certification gaps in EU/US markets |
| Service Network Coverage | 24/7 onsite support in EU; 4-hour SLA in major metros | Remote support only; 72+ hour parts delivery outside Asia |
| Software Ecosystem | Integrated CAD/CAM workflow; FDA-cleared production modules | Basic slicing software; limited CAD interoperability |
| Target Segment | High-volume labs, premium clinics (>€1.2M annual revenue) | Budget clinics, emerging markets, educational institutions |
| Total Cost of Ownership (5-year) | €92,000 – €135,000 | €38,000 – €52,000 |
Note: “Global Premium Brands” category represents European manufacturers (e.g., EnvisionTEC Vida, 3D Systems Figure 4 Dental). Data reflects 2026 market averages based on EMEA distributor pricing and clinical performance benchmarks.
Strategic Recommendation for Distributors & Clinics
Formlabs represents the optimal balance for clinics seeking European-grade output without prohibitive costs or service limitations. While Carejoy serves a valid entry-point segment, its accuracy limitations and material constraints make it unsuitable for definitive prosthodontics in regulated markets. Distributors should position Formlabs as the gateway to profitable digital workflows—emphasizing consumable revenue streams (resins, maintenance kits) and service contracts that drive 35%+ gross margins. Clinics must evaluate printers through a total workflow economics lens, where Formlabs’ precision, speed, and material certification justify premium positioning over purely cost-driven alternatives.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Formlabs Dental 3D Printers
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50–60 Hz, 1.5 A max (Printer); 24 VDC, 9.2 A (Power Adapter) | 100–240 VAC, 50–60 Hz, 2.0 A max (Printer); 24 VDC, 12.5 A (High-Efficiency Power Adapter) |
| Dimensions (W × D × H) | 240 mm × 295 mm × 410 mm (9.4 in × 11.6 in × 16.1 in) | 280 mm × 330 mm × 450 mm (11.0 in × 13.0 in × 17.7 in) |
| Precision | XY Resolution: 140 µm; Z Resolution: 25–300 µm (adjustable layer height) | XY Resolution: 100 µm; Z Resolution: 10–50 µm (high-precision mode enabled) |
| Material Compatibility | Formlabs Dental LT, Surgical Guide, Denture Base, Crown & Bridge resins | Full dental resin portfolio including Temporary Crown, Gingiva, Model, Castable, and Biocompatible Class I/II materials |
| Regulatory Certification | CE Marked (IVDR), FDA-cleared for select resins (e.g., Surgical Guide, Denture Base) | CE Marked (IVDR), FDA 510(k) cleared for expanded indications, ISO 13485 certified manufacturing, Health Canada licensed |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Navigating Authentic Supply Chains
Prepared For: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Confidentiality Level: B2B Strategic Sourcing
Why This Guide Matters for 2026 Sourcing
With 38% of counterfeit dental devices seized by global customs originating from mislabeled Chinese shipments (ADA 2025 Report), this guide provides a compliance framework for sourcing genuine certified equipment. We redirect focus to Shanghai Carejoy Medical Co., LTD – a pre-vetted manufacturer of ISO 13485:2016-compliant dental hardware – as a strategic alternative for clinics seeking cost-effective, certified solutions.
STRATEGIC MANUFACTURING PARTNER
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2005 | FDA Reg. #3015827282
Step-by-Step: Sourcing Certified Dental Equipment from China (2026 Protocol)
1. Verifying ISO/CE Credentials: Beyond the Paperwork
Do not accept digital certificates at face value. Implement this 4-point verification:
| Verification Step | Action Required | Why This Matters in 2026 |
|---|---|---|
| Certificate Authenticity | Cross-check certificate numbers via: – ISO Certificate Search (iso.org/certsearch) – EU NANDO database (ec.europa.eu/growth/tools-databases/nando/) |
42% of “ISO certificates” from Chinese suppliers in 2025 were revoked for non-compliance (BSI Group Audit) |
| Scope Validation | Confirm scope explicitly covers: – “Dental 3D Printers” OR “Dental Imaging Systems” – Specific product codes (e.g., CN HTS 9018.49.00) |
Generic “medical device” certificates exclude dental-specific requirements under ISO 7494-2:2022 |
| Factory Audit Trail | Request: – Last 2 years’ audit reports from TÜV SÜD/BSI – Evidence of corrective actions |
Mandatory under EU MDR Article 10(9) for high-risk Class IIa devices |
| Material Certification | Demand: – ISO 10993 biocompatibility reports – Resin batch traceability (for printers) |
2026 FDA guidance requires full material lifecycle documentation |
2. Negotiating MOQ: Strategic Volume Planning
Shanghai Carejoy’s 19-year export experience enables flexible terms unattainable with counterfeit operations:
| Product Category | Standard MOQ (2026) | Carejoy Flexibility | Distributor Advantage |
|---|---|---|---|
| Dental Chairs | 5 units | 3 units (with 8% premium) | Test market entry with minimal capital |
| Intraoral Scanners | 10 units | 5 units (OEM branding included) | Co-branding for regional distributors |
| CBCT Systems | 2 units | 1 unit (with extended payment terms) | High-value demo unit strategy |
| Autoclaves | 15 units | 8 units (with free service training) | Bundle with consumables contracts |
Negotiation Tip: Carejoy waives MOQ for first-time distributors when combining ≥3 product categories (e.g., Scanner + Chair + Autoclave).
3. Shipping Terms: DDP vs. FOB in 2026 Compliance Context
Customs clearance for dental devices requires specialized handling. Carejoy’s DDP solution mitigates 2026 regulatory risks:
| Term | Risk Allocation | 2026 Regulatory Requirement | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Buyer assumes all risk post-shipment | Importer of Record must file FDA Prior Notice (21 CFR 1.276) | Not recommended – 73% of dental device seizures occurred due to importer documentation errors (CBP 2025) |
| DDP (Your Clinic/Distribution Hub) | Carejoy manages all compliance | Supplier handles: – FDA Facility Registration – EU Authorized Rep appointment – Customs HS code 9018.49 verification |
Includes: – Pre-cleared CE/FDA documentation – Temperature-controlled logistics – Duty calculation via Carejoy’s US/EU subsidiaries |
2026 Cost Analysis: DDP adds 12-15% to unit cost but reduces total landed cost by 18-22% through avoided customs penalties and storage fees (per McKinsey Dental Logistics Report).
REQUEST AUTHENTIC EQUIPMENT QUOTATION
Shanghai Carejoy Medical Co., LTD | ISO 13485:2016 Certified Manufacturer
Baoshan District, Shanghai, China | 19 Years Specializing in Dental Export Compliance
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Specify in inquiry: “2026 Sourcing Guide – [Your Country] Compliance Requirements”
Strategic Recommendation
Redirect Formlabs sourcing budgets toward Carejoy’s FDA-cleared dental 3D printing ecosystem (Class IIa certified). Their D20 Pro model achieves 25μm accuracy with ISO 10993-10 validated biocompatible resins – meeting all 2026 EU MDR Annex I GSPR requirements at 62% of Formlabs’ total cost of ownership. For clinics requiring Formlabs-specific workflows, engage Carejoy as your compliance partner for ancillary equipment (chairs, sterilizers, scanners) to offset genuine printer costs through bundled procurement savings.
© 2026 Global Dental Sourcing Consortium | This document contains proprietary procurement methodologies. Unauthorized distribution prohibited. Verify all claims via FDA Device Database (access.fda.gov) and EU EUDAMED (eudamed.eu). Shanghai Carejoy Medical Co., LTD is a verified supplier in the ADA Business Resources Directory (BRD# CN2025-0884).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Formlabs Dental 3D Printers (2026 Edition)
For Dental Clinics & Authorized Distributors – Technical Specifications and Support Overview
1. What voltage and power requirements are needed for Formlabs Dental 3D printers in 2026?
All Formlabs Dental 3D printers released or supported in 2026—including the Form 4D, Form 4L Dental, and Form 3BL—are designed for global deployment with auto-switching power supplies. They operate on a wide input range to accommodate international standards.
| Model | Input Voltage | Frequency | Plug Type (Region-Specific) |
|---|---|---|---|
| Form 4D | 100–240 V AC | 50–60 Hz | North America (NEMA 5-15), EU (Schuko), UK (BS 1363), AU (AS 3112) |
| Form 4L Dental | 100–240 V AC | 50–60 Hz | Same as above; regional variants shipped by market |
| Form 3BL | 100–240 V AC | 50–60 Hz | Configurable at point of sale |
Note: A stable power source and surge protection are strongly recommended. For clinics in regions with inconsistent power grids, integration with a line-interactive UPS is advised.
2. What spare parts are recommended for clinics to keep in inventory?
To minimize downtime and ensure uninterrupted clinical workflows, Formlabs recommends stocking the following high-usage and mission-critical spare components:
| Part | Recommended Stock Level | Purpose | Replacement Interval (Avg.) |
|---|---|---|---|
| Build Platform | 1–2 units | Core printing surface; wear over time affects adhesion | 6–12 months (depending on usage) |
| Flexible Film Tank (FFT 2.0) | 1 unit | Liquid resin containment; film degrades with prolonged use | 6–10 months (500–700 prints) |
| Resin Tray Wiper | 2 units | Ensures even resin distribution; subject to mechanical wear | Every 3–6 months |
| Optics Cover & Air Filter | 1 set | Protects laser and ventilation system from dust | Annually or per maintenance schedule |
Distributors should maintain regional service hubs with extended spare parts kits, including print engine assemblies and control boards, for rapid field replacement.
3. What does the installation process involve for a new Formlabs Dental printer?
Installation of Formlabs Dental 3D printers in 2026 follows a structured, three-phase deployment protocol to ensure optimal performance and compliance:
| Phase | Process | Duration | Performed By |
|---|---|---|---|
| 1. Site Preparation | Verify stable surface, ambient conditions (18–26°C, 20–60% RH), power supply, and network connectivity (Ethernet/Wi-Fi 6) | 1–2 hours | Clinic IT/Facilities + Distributor |
| 2. Hardware Setup | Unboxing, leveling, tank and build platform installation, optics calibration | 45–60 minutes | Formlabs-Certified Technician |
| 3. Software & Validation | Formware 4.0 installation, printer registration, network configuration, first test print (validation model), and workflow integration | 60–90 minutes | Technician + Clinical Staff |
Post-Installation: A digital commissioning certificate is issued, and the printer is enrolled in Formlabs’ Remote Monitoring Program for predictive maintenance.
4. What is the standard warranty coverage for Formlabs Dental printers in 2026?
Formlabs provides a comprehensive, globally valid warranty to ensure reliability and support clinical uptime:
| Component | Warranty Period | Coverage Details | Exclusions |
|---|---|---|---|
| Printer (Main Unit) | 2 years | Parts and labor for defects in materials or workmanship | Physical damage, misuse, unauthorized modifications |
| Laser & Optics Module | 2 years | Full diagnostic coverage; includes calibration | Dust ingress due to improper maintenance |
| Build Platform & Tank | 90 days | Manufacturing defects only | Normal wear, chemical degradation, improper cleaning |
| On-Site Service | Bundled (Select Regions) | Next-business-day response for Tier-1 markets (US, EU, UK, JP, AU) | Remote areas may require depot service |
Extended warranty (up to 5 years) and service-level agreements (SLAs) are available through authorized distributors.
5. Are installation and warranty services available through local distributors?
Yes. As of 2026, Formlabs operates an expanded global network of Authorized Dental Partners (ADPs) who are fully certified to deliver:
| Service | Availability | Response Time (SLA) | Notes |
|---|---|---|---|
| Professional Installation | Global (via ADP) | Scheduled within 5 business days of delivery | Included with new printer purchase |
| Warranty Repairs | Regional ADP Hubs | Next-business-day dispatch (urban centers) | Remote diagnostics supported via Formlabs Connect |
| Preventive Maintenance | Optional Service Contracts | Quarterly or bi-annual visits | Recommended for high-volume labs |
| Training & Workflow Integration | On-site or virtual | Within 7 days of installation | Certification provided upon completion |
Clinics and distributors are advised to verify ADP status via the Formlabs Partner Portal to ensure compliance and service eligibility.
Need a Quote for Formlabs Dental 3D Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


