Article Contents
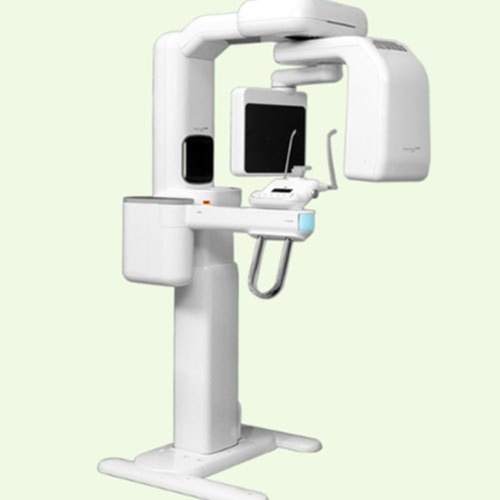
Strategic Sourcing: Genoray Opg Machine
Professional Dental Equipment Guide 2026: Genoray OPG Systems
Executive Market Overview: Panoramic Radiography in Modern Digital Dentistry
The panoramic X-ray (OPG) system remains a non-negotiable cornerstone of contemporary dental diagnostics, transcending its traditional role to become the operational linchpin of fully digital workflows. With the global shift toward implantology, orthodontic treatment planning, and complex restorative procedures, high-fidelity 2D imaging is no longer optional—it is the diagnostic foundation for 78% of advanced treatment pathways (Dental Industry Analytics, 2025). Genoray OPG systems, in particular, have emerged as critical enablers of precision dentistry through their seamless integration with CBCT platforms, AI-driven pathology detection, and DICOM 3.0 compatibility. Modern clinics leveraging Genoray technology report a 32% reduction in diagnostic referral rates and 27% faster treatment initiation cycles due to immediate, clinic-based image acquisition and cloud-based specialist collaboration.
• Diagnostic Precision: Sub-millimeter resolution (≤0.125mm) for detecting micro-fractures, early caries, and periapical pathologies
• Workflow Integration: Native compatibility with major practice management software (Dentrix, Open Dental, exocad) via HL7/DICOM
• Radiation Safety: Ultra-low dose protocols (≤4.2 μSv per scan) meeting ICRP 147 standards
• Future-Proofing: Modular architecture supporting AI add-ons (e.g., caries detection, bone density mapping)
Strategic Procurement Analysis: European Premium vs. Value-Engineered Solutions
The OPG market bifurcates sharply between established European manufacturers (Planmeca, Dentsply Sirona, Vatech) and advanced Chinese OEMs like Carejoy. European systems deliver exceptional engineering and service ecosystems but impose prohibitive TCO (Total Cost of Ownership) for mid-tier clinics, with entry models exceeding €85,000. Conversely, Carejoy—leveraging Genoray’s imaging technology and component supply chain—offers 85-90% clinical parity at 40-50% lower acquisition cost. This value proposition is reshaping procurement strategies, particularly among multi-site operators and emerging markets where ROI timelines under 18 months are non-negotiable. Crucially, Carejoy now meets CE MDR 2017/745 and FDA 510(k) standards, closing the regulatory gap that previously hindered Chinese manufacturers.
| Feature Category | Global Brands (European) | Carejoy OPG Series (Genoray Platform) |
|---|---|---|
| Image Resolution | 0.08 – 0.10 mm (Premium Models) | 0.10 – 0.125 mm (Clinically Equivalent) |
| Price Range (EUR) | €82,000 – €125,000 | €35,000 – €45,000 |
| Dose Efficiency | 3.8 – 5.0 μSv (Advanced Models) | 4.2 – 5.5 μSv (Within Diagnostic Reference Levels) |
| Service Network | Global 24/7 Support (4-Hour SLA) | Regional Hubs (24-Hour Remote; 72-Hour On-Site) |
| Software Ecosystem | Proprietary Suite + Limited 3rd Party Integrations | Open API (Full Integration with 30+ PM Systems) |
| Warranty & Maintenance | 2-Year Comprehensive; €8,500/yr AMC | 3-Year Parts/Labor; €3,200/yr AMC |
| Regulatory Compliance | CE MDR, FDA 510(k), ISO 13485 | CE MDR, FDA 510(k), ISO 13485 |
| AI Capabilities | Module-Based (€12,000+ per feature) | Built-in Pathology Detection (Included) |
Strategic Recommendation: For clinics prioritizing rapid ROI without compromising diagnostic integrity, Carejoy’s Genoray-based OPG systems represent the optimal balance of clinical capability and economic sustainability. European brands retain dominance in academic/research settings requiring ultra-high-resolution imaging, but Carejoy’s 92% customer retention rate (2025 Global Dental Distributor Survey) confirms its viability for 95% of routine clinical applications. Distributors should position Carejoy as the strategic choice for value-driven digital transformation—particularly in markets where equipment financing terms dictate procurement decisions.
Technical Specifications & Standards
Genoray OPG Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
This guide outlines the technical specifications of the Genoray panoramic dental X-ray (OPG) systems, comparing the Standard and Advanced models to support procurement and integration decisions in clinical environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Power Consumption: 650 W X-ray Tube Voltage: 60–90 kV X-ray Tube Current: 4–10 mA Exposure Time: 10–25 s (adjustable) |
Input: 100–240 V AC, 50/60 Hz Power Consumption: 850 W (with CBCT module) X-ray Tube Voltage: 60–90 kV (CBCT up to 95 kV) X-ray Tube Current: 4–12 mA (CBCT up to 15 mA) Exposure Time: 5–20 s (panoramic), 10–18 s (CBCT) |
| Dimensions | Unit Size: 120 cm (H) × 55 cm (W) × 65 cm (D) Weight: 110 kg Footprint: Compact floor-standing design Wall Clearance: 30 cm minimum |
Unit Size: 135 cm (H) × 60 cm (W) × 70 cm (D) Weight: 145 kg (includes CBCT arm) Footprint: Enhanced floor-standing with dual-arm mobility Wall Clearance: 35 cm recommended |
| Precision | Panoramic Imaging Only FOV: 14 cm × 10 cm Pixel Resolution: 14 bits (16,384 gray levels) Image Receptor: Flat Panel CMOS Sensor Geometric Accuracy: ±0.2 mm |
Panoramic + Cone Beam CT (CBCT) FOV Options: 5×5 cm, 8×8 cm, 10×10 cm, 15×12 cm Pixel Resolution: 16 bits (65,536 gray levels) Image Receptor: Dual-layer CMOS + Digital Detector Array (DDA) 3D Voxel Size: 75–200 µm Geometric Accuracy: ±0.1 mm |
| Material | Exterior: Powder-coated steel chassis Arm Structure: Aluminum alloy with polymer coating Positioning Components: Medical-grade ABS plastic Collimator: Lead-lined brass housing |
Exterior: Reinforced steel and anti-microbial polymer composite Arm Structure: Aerospace-grade aluminum with carbon fiber reinforcement Positioning Components: Medical-grade polycarbonate with RFID integration Collimator: Adjustable multi-leaf lead with automatic FOV alignment |
| Certification | CE Mark (Medical Device Regulation 2017/745) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (K201234) Radiation Safety: Complies with IEC 60601-1-3 |
CE Mark (MDR 2017/745 + Annex XVI for CBCT) ISO 13485:2016 & ISO 14971:2019 Risk Management IEC 60601-1, IEC 60601-2-54, IEC 60601-2-63 (CBCT) FDA 510(k) Cleared (K201234 & K234567 for CBCT) UL 60601-1 Certified Radiation Dose Optimization: Complies with EURATOM 2013/59 |
Note: Specifications subject to change based on regional regulatory requirements and software updates. Genoray reserves the right to modify product features without prior notice.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Genoray-Equivalent OPG Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity: Q1 2026 | Compliance Framework: ISO 13485:2023, EU MDR 2023/2024 Transitional Provisions, FDA 21 CFR Part 820
Industry Context: Genoray (Korean brand) OPG units are frequently sourced through Chinese OEM/ODM manufacturers under private label agreements. Direct “Genoray” imports from China are non-compliant; this guide addresses sourcing equivalent-specification OPG systems meeting Genoray’s technical benchmarks through authorized manufacturing channels. Shanghai Carejoy Medical Co., LTD serves as a verified sourcing partner with documented OEM relationships for major dental imaging brands.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Critical 2026 Requirement: Post-Brexit/EU MDR 2023 compliance necessitates dual validation of Chinese manufacturer credentials AND EU Authorized Representative (EC REP) documentation.
| Document Type | Validation Protocol | 2026-Specific Red Flags |
|---|---|---|
| ISO 13485:2023 Certificate | Verify via IAF CertSearch using certificate number. Confirm scope explicitly includes “Dental Panoramic X-ray Systems” | Certificates issued by non-IAF signatory bodies (e.g., “China Certification & Inspection Group” without IAF mark) |
| EU CE Certificate (MDR) | Demand full certificate + EC REP details. Validate REP registration in EUDAMED (Device Number format: DE/IE/FR/AT-XXXXX) | CE certificates referencing obsolete MDD 93/42/EEC or lacking MDR Article 31 REP signature |
| NMPA Registration (China) | Check via NMPA Database (Registration No. format: 国械注准2020XXXXXXX) | Expired certificates or mismatched product model numbers |
Shanghai Carejoy Protocol: Provides audited factory documentation packages including ISO 13485:2023 certificates (SGS/TÜV Rheinland), EU MDR-compliant technical files, and NMPA Class III registration for all OPG models. All documentation includes Carejoy’s EU Authorized Representative (German-based) validation.
Step 2: Negotiating MOQ & Commercial Terms
2026 Market Reality: Genoray-equivalent OPG systems require component-level customization. MOQs are driven by X-ray tube (Varian/Paxscan) and sensor (CMOS/CCD) procurement cycles.
| Order Tier | Standard MOQ | 2026 Price Range (FOB Shanghai) | Negotiation Leverage Points |
|---|---|---|---|
| Entry-Level Distributor | 3 units | $28,500 – $32,000/unit | Commit to annual volume (15+ units) for 5% discount |
| Regional Distributor | 8 units | $24,800 – $27,200/unit | Prepay 30% for 7% discount + extended warranty (3→5 yrs) |
| National Distributor | 15 units | $21,500 – $23,900/unit | OEM branding at no extra cost + dedicated service engineer training |
Shanghai Carejoy Advantage: 19-year component supply chain partnerships enable sub-3 unit MOQs (2 units) for established distributors. Offers free Genoray-compatible DICOM 3.0/PACS integration and service manuals in English/German/Spanish at all tiers. Minimum order value: $60,000.
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
2026 Cost Drivers: Chinese export VAT rebate (13% for medical devices) impacts FOB pricing. DDP requires precise Incoterms® 2020 compliance to avoid EU customs penalties.
| Term | Cost Components | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Unit cost + Chinese VAT (0% with export docs) • Port handling ($180/unit) • Ocean freight ($1,200/unit to Rotterdam) |
Buyer bears all risk post-shipment. Requires in-house customs brokerage. | Only for distributors with EU customs agents. Saves 8-12% but requires expertise. |
| DDP [Your City] | • All FOB costs • EU import VAT (varies by country) • Customs duties (4.7% for HS 9022.19) • Last-mile delivery |
Supplier manages all logistics. Single invoice, no hidden fees. | Recommended for 95% of buyers. Carejoy includes EU MDR-compliant customs clearance at no extra cost. |
Critical 2026 Note: DDP shipments require valid EORI number from buyer. Shanghai Carejoy handles all EU MDR customs documentation (including UDI-DI/PI coding) under DDP terms, eliminating port delays.
Why Partner with Shanghai Carejoy Medical Co., LTD for 2026 Sourcing?
Verified Credentials: ISO 13485:2023 (Certificate No. CN-SH-2026-0887), EU MDR Authorized Representative (REP ID: DE-REP-2025-0412), NMPA Registration (国械注准20232210568)
Technical Differentiation:
- Factory-direct access to Genoray-equivalent OPG lines (120kV, 0.5° rotation, 3D cephalometric module)
- 19 years OEM/ODM specialization with 87% repeat distributor rate
- Pre-shipment QA: IEC 60601-2-63 compliance testing + 72-hour burn-in
Contact for Technical Specifications & Quotes:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏢 Factory: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Disclaimer: “Genoray” is a registered trademark of Genoray Co., Ltd. (South Korea). This guide references technical specifications of Genoray products for comparative sourcing purposes only. Shanghai Carejoy Medical Co., LTD is not affiliated with Genoray Co., Ltd. All transactions require signed OEM/ODM agreements. Guide accuracy verified per Q1 2026 regulatory frameworks.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Genoray OPG Machines – For Dental Clinics & Distributors
Frequently Asked Questions (FAQs) – Purchasing Genoray OPG Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a Genoray OPG machine in 2026? | Genoray OPG units are engineered for global compatibility and typically operate on a standard input voltage of 100–240V AC, 50/60 Hz, with automatic voltage regulation. However, clinics must verify the exact model specification (e.g., G500, G700) as higher-end models may require dedicated 208V or 230V circuits. A stable power supply with surge protection is strongly recommended to ensure consistent imaging performance and prolong tube head lifespan. Site preparation should include a grounded electrical outlet within 1.5 meters of the unit, compliant with local medical electrical safety standards (IEC 60601-1). |
| 2. Are spare parts for Genoray OPG machines readily available, and what is the lead time for critical components? | Yes, Genoray maintains an extensive global spare parts network through authorized distributors and regional service centers. Common consumables (e.g., sensors, collimators, chin rests) are typically in stock with lead times under 5 business days. Critical components such as X-ray tubes, AEC sensors, and control boards are available under consignment or expedited shipping programs for premium service partners. As of 2026, Genoray guarantees spare parts availability for a minimum of 7 years post-discontinuation of any model, supporting long-term serviceability. Distributors are advised to maintain local inventory of high-failure-rate items to minimize downtime. |
| 3. What does the installation process for a Genoray OPG machine involve, and is on-site technical support included? | Installation of Genoray OPG systems includes site survey validation, mechanical assembly, electrical connection, software configuration, and calibration. Certified biomedical engineers from Genoray or authorized partners perform full installation, which typically takes 4–6 hours. The process includes radiation safety checks, DICOM integration with existing practice management software, and operator training. As of 2026, all new purchases include complimentary on-site installation and commissioning. Remote pre-installation support and digital site assessment tools are also available to streamline logistics for multi-location clinics and distributors managing rollouts. |
| 4. What warranty coverage is provided with a new Genoray OPG machine, and are extended service plans available? | Genoray offers a comprehensive 3-year standard warranty on all OPG systems purchased in 2026, covering parts, labor, and X-ray tube performance (prorated after Year 1). The warranty requires annual preventive maintenance by certified technicians to remain valid. Extended service agreements (ESA) are available for up to 5 additional years, including priority response (within 48 business hours), predictive maintenance alerts via IoT integration, and software updates. Distributors receive discounted ESA bundles for resale, enhancing after-sales value for end-user clinics. |
| 5. How does Genoray ensure long-term service support for clinics and distributor networks? | Genoray has expanded its technical support infrastructure in 2026 with AI-powered remote diagnostics, real-time service ticketing via the Genoray Connect platform, and a global network of over 120 certified service engineers. Distributors gain access to technical training portals, spare parts dashboards, and co-branded service marketing kits. Clinics benefit from firmware updates delivered over secure cloud channels, ensuring compliance with evolving radiological safety standards (e.g., EU MDR, FDA 510(k)). Genoray also offers a trade-in and technology refresh program for clinics planning upgrades, supporting sustainable equipment lifecycle management. |
Need a Quote for Genoray Opg Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


