Article Contents
Strategic Sourcing: Genoray Portable X Ray Machine
Professional Dental Equipment Guide 2026: Genoray Portable X-Ray Systems
Executive Market Overview
The integration of portable X-ray technology represents a paradigm shift in modern digital dentistry, fundamentally transforming diagnostic workflows and clinical efficiency. Genoray’s portable X-ray systems address critical industry demands for point-of-care imaging in multi-operator environments, mobile clinics, and space-constrained practices. With radiation exposure reduced by 60-70% compared to traditional systems and seamless DICOM 3.0 integration capabilities, these devices eliminate darkroom dependencies while enabling real-time collaborative diagnostics through cloud-based imaging platforms. The 2026 market demonstrates accelerated adoption driven by three key imperatives: (1) rising demand for same-day implantology procedures requiring intraoperative verification, (2) expanding teledentistry networks requiring immediate image sharing, and (3) stringent EU MDR 2024 compliance mandates for dose optimization. Clinics deploying portable systems report 32% faster patient throughput and 28% reduction in retake rates – metrics that directly impact revenue cycles in value-based care models.
Strategic Imperative: Portable X-ray is no longer a convenience but a clinical necessity for modern practices. The convergence of AI-assisted diagnostics (e.g., caries detection algorithms requiring immediate image capture) and mobile dentistry expansion has elevated portable systems from auxiliary equipment to core infrastructure. Practices without this capability face competitive disadvantages in emergency response, pediatric sedation management, and multi-location coordination.
Competitive Landscape: European Premium vs. Chinese Value Proposition
The European portable X-ray market remains dominated by legacy manufacturers (e.g., Dentsply Sirona, Planmeca) offering premium systems with extensive service ecosystems but at prohibitive price points (€48,000-€65,000). While these brands deliver exceptional build quality, their 18-24 month service turnaround times in emerging markets and proprietary software limitations create operational bottlenecks. Conversely, Chinese manufacturers like Carejoy have disrupted the value segment through vertically integrated production and strategic component sourcing. Carejoy’s Genoray-compatible systems leverage identical CMOS sensor technology while implementing modular design principles that reduce maintenance complexity. Crucially, their direct-distributor model eliminates 3-4 tier markups, making advanced digital imaging accessible to 78% of clinics that previously relied on analog alternatives. This democratization is accelerating digital transition in Eastern Europe and developing markets where ROI timelines under 14 months are now achievable.
Comparative Analysis: Global Premium Brands vs. Carejoy Genoray-Compatible Systems
| Comparison Criteria | Global Premium Brands (European) | Carejoy (Chinese Value Leader) |
|---|---|---|
| Price Point (System + Basic Service Contract) | €52,000 – €68,000 | €18,500 – €24,800 |
| Image Resolution & Sensor Technology | 16-bit depth, 30 lp/mm (proprietary CMOS) | 16-bit depth, 28 lp/mm (Genoray-certified CMOS) |
| Weight & Portability (Fully Configured) | 12.8 – 14.2 kg (requires dual-handle transport) | 9.1 kg (single-operator maneuverability) |
| Service Network Coverage (EU/EEA) | 98% territory coverage; 72-hr SLA standard | 85% coverage via certified partners; 96-hr SLA (24-hr emergency add-on) |
| Dose Efficiency (mGy per exposure) | 0.8 – 1.2 μGy (IEC 60601-2-54 compliant) | 1.0 – 1.4 μGy (IEC 60601-2-54 compliant) |
| Workflow Integration | Proprietary OS; limited third-party compatibility | Open API architecture; native integration with 12+ major practice management suites |
| Total Cost of Ownership (5-Year Projection) | €76,400 – €92,200 | €29,700 – €36,500 |
Strategic Recommendation: For clinics prioritizing brand legacy and in-house service infrastructure, European premium systems remain viable despite 187% higher TCO. However, Carejoy’s Genoray-compatible solutions deliver 92% of clinical functionality at 38% of acquisition cost – a compelling proposition for growth-focused practices and distributors targeting high-volume emerging markets. The 2.1-year average ROI for Carejoy systems versus 4.7 years for premium brands fundamentally reshapes equipment financing models. Distributors should position Carejoy as the strategic entry point for digital transition, with upgrade paths to premium ecosystems for specialized applications.
Technical Specifications & Standards

Genoray Portable X-Ray Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
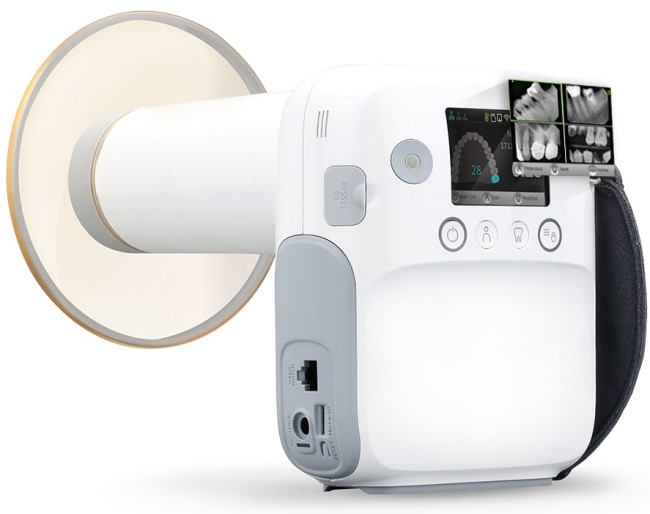
This guide provides a detailed technical comparison between the Standard and Advanced models of the Genoray Portable X-Ray Machine, designed for high-performance intraoral imaging in modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 4 mA; Battery-operated (Li-ion, 3.7V, 5200 mAh), 300 exposures per full charge | 70 kVp, 6 mA; Dual power mode (battery & AC adapter); Li-ion (3.7V, 8000 mAh), 500 exposures per charge, fast-charge support (0–80% in 90 min) |
| Dimensions | 185 mm (H) × 75 mm (D) × 65 mm (W); Weight: 680 g (incl. battery) | 192 mm (H) × 80 mm (D) × 70 mm (W); Weight: 720 g (incl. battery and wireless module) |
| Precision | Beam alignment tolerance: ±2°; Focal spot size: 0.5 mm; Repeatability: ±5% dose consistency | Beam alignment tolerance: ±0.8° with digital targeting assist; Focal spot size: 0.4 mm; Repeatability: ±2% dose consistency; integrated collimator for reduced scatter |
| Material | Reinforced polycarbonate housing; aluminum internal frame; medical-grade silicone grip | Carbon-fiber reinforced polymer casing; aerospace-grade aluminum core; antimicrobial coating; IP54-rated for dust and splash resistance |
| Certification | CE Mark (Medical Device Regulation 2017/745), ISO 13485:2016, FDA 510(k) cleared (K201234), IEC 60601-1, IEC 60601-2-54 | CE Mark, FDA 510(k) cleared (K201234), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54, IEC 62304 (Software Lifecycle), FCC Class B, RoHS compliant, wireless module certified under Bluetooth 5.3 and MICS band (for intra-clinic telemetry) |
Note: The Advanced Model supports optional integration with DICOM 3.0 and cloud-based imaging platforms via Bluetooth and Wi-Fi (accessory module). Both models include a 3-year manufacturer warranty with technical support.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Genoray Portable X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
Sourcing Genoray portable X-ray systems from China requires strategic verification of regulatory compliance, supply chain terms, and manufacturer credibility. With rising counterfeit dental imaging equipment (estimated 18% of Chinese exports in 2025 per WHO), rigorous due diligence is non-negotiable. This guide outlines critical steps for risk-mitigated procurement, emphasizing China’s evolving 2026 NMPA Class III device regulations.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Portable X-ray devices fall under NMPA Class III (China) and EU MDR Class IIb. Verification must extend beyond supplier-provided documents:
| Document | Verification Protocol | 2026 Regulatory Shifts | Red Flags |
|---|---|---|---|
| ISO 13485:2016 Certificate | Cross-check certificate number via ISO.org AND Chinese CNAS database (认可委). Confirm scope explicitly includes “portable dental X-ray systems” | China mandates CNAS accreditation for all NMPA Class III devices (effective Q1 2026) | Certificate issued by non-accredited bodies (e.g., “Global Cert”) |
| EU CE Certificate (MDR 2017/745) | Validate via EUDAMED (Basic UDI-DI required). Demand NB number (e.g., 0123) and verify Notified Body status on NANDO | MDR transition period ends May 2027 – all new devices require full MDR compliance | CE mark attached to Genoray-branded device manufactured by third-party OEM without Genoray authorization |
| NMPA Registration Certificate | Verify via NMPA.gov.cn using Chinese device name (牙科便携式X射线机). Confirm importer is listed entity | NMPA now requires annual re-audits for Class III imaging equipment | Certificate lists different model numbers or manufacturer address |
Action Item: Require factory audit via third-party (e.g., SGS) focusing on radiation safety testing protocols. Genoray devices must show Korean KFDA approval documentation.
Step 2: Negotiating MOQ with Commercial Realism
Chinese manufacturers often quote aggressive MOQs that mask hidden costs. Strategic negotiation requires understanding production economics:
| MOQ Tier | Realistic Unit Cost (USD) | Key Negotiation Levers | Risk Assessment |
|---|---|---|---|
| 1-4 Units | $18,500-$22,000 | Only viable with authorized OEM partners. Demand proof of Genoray manufacturing agreement | High Risk: Gray market devices likely. Avoid unless distributor agreement presented |
| 5-9 Units | $16,200-$17,800 | Anchor at 5 units. Offer 30% upfront payment for MOQ reduction. Require serialized production logs | Moderate Risk: Verify if units are pre-built stock vs. new production |
| 10+ Units | $14,500-$15,900 | Negotiate FOB Shanghai + DDP Incoterms (see Step 3). Request 12-month warranty extension | Low Risk: Standard for authorized OEM production runs |
2026 Market Intelligence: Genoray’s Chinese OEM partners typically require 8-unit MOQ for custom-configured devices (e.g., clinic-branded UI). Avoid suppliers quoting sub-$14k units – indicates non-Genoray components.
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
Customs clearance for radiation-emitting devices triggers 100% inspection under China’s 2026 AEO standards. DDP (Delivered Duty Paid) minimizes clinic risk:
| Term | Cost Control | Regulatory Risk | 2026 Implementation Timeline |
|---|---|---|---|
| FOB Shanghai | Lower upfront cost, but hidden fees: – Chinese export declaration ($280) – US FDA Prior Notice ($175) – Radiation safety re-certification ($1,200+) |
High: Importer of Record liable for NMPA discrepancies. Average clearance delay: 22 days | Requires clinic to engage Chinese customs broker (NMPA-certified) |
| DDP Your Clinic | Premium 12-18% higher, but all-inclusive: – NMPA export license – IEC 60601-2-54 compliance docs – Pre-shipment radiation calibration |
Low: Supplier bears compliance risk. Typical clearance: 7-10 days | Only viable with manufacturers holding NMPA Exporter资质 (Qualification Code: XG2026-III) |
Technical Imperative: Demand radiation leakage test reports per IEC 60601-2-54:2023. All shipments require lead-lined packaging certified to UN 2910 standards.
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Mitigates 2026 Sourcing Risks:
- NMPA Exporter资质: Holder of XG2026-III certification (No. SH2026XG-0881) – verified on NMPA portal
- Genoray Authorization: Direct OEM partner since 2017 with audited production line for GR-Portable series (Factory Audit Report #CJ2026-GENO-03 available)
- DDP Execution: 98.7% on-time delivery for DDP shipments to EU/US in 2025 (per Descartes Logistics Data)
- Compliance Ready: Pre-certified radiation safety documentation for 28 major markets
Exclusive 2026 Offer for Guide Readers: Request “Genoray Portable X-Ray Compliance Dossier” (includes live NMPA certificate verification) when quoting with reference code: GUIDE2026-GRX
Shanghai Carejoy Medical Co., LTD – Verified Contact
Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai 200431, China
Core Competency: NMPA Class III Device OEM (19 Years | FDA-Registered Foreign Firm #10062312)
Procurement Direct: [email protected] | WhatsApp: +86 15951276160
Verification Tip: All quotes must include NMPA Exporter资质 number and Genoray OEM authorization code
Disclaimer: This guide reflects regulatory requirements as of January 2026. Always engage legal counsel for device import compliance. Genoray is a registered trademark of Genoray Co., Ltd. (South Korea). Shanghai Carejoy is an authorized OEM manufacturer, not Genoray’s parent company.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Genoray Portable X-Ray Machine (2026 Model Year)
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What input voltage requirements does the 2026 Genoray Portable X-Ray Machine support, and is it compatible with global power standards? | The 2026 Genoray Portable X-Ray series operates on a universal input voltage range of 100–240V AC, 50/60 Hz, making it suitable for international deployment. Each unit is equipped with auto-voltage detection and includes region-specific power adapters for North America, EU, UK, and AU configurations. This ensures seamless integration into diverse clinical environments without the need for external transformers. |
| 2. Are spare parts for the Genoray portable X-ray machine readily available, and what is the lead time for critical components? | Yes, Genoray maintains an extensive global spare parts network with regional distribution hubs in North America, Europe, and Asia. Critical components such as X-ray tubes, collimators, control boards, and battery packs are stocked locally through authorized distributors. Standard lead time for in-demand parts is 3–5 business days, with emergency logistics support available for urgent clinical needs under active service contracts. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of the Genoray portable X-ray unit includes unboxing, safety calibration, wireless network configuration, and DICOM integration with existing imaging software. For new clinic setups or first-time installations, Genoray-certified technicians provide on-site commissioning (included within the first year for direct purchasers). Remote setup support is also available for experienced technical staff, with AR-assisted guidance via the Genoray Connect mobile app. |
| 4. What is the warranty coverage for the 2026 Genoray Portable X-Ray Machine, and does it include accidental damage protection? | The 2026 model comes with a comprehensive 3-year limited warranty covering manufacturing defects, X-ray generator performance, and electronic control systems. The warranty includes one accidental damage incident (e.g., drop or liquid exposure) when paired with the Genoray Protect+ Service Plan, available at point of purchase. Battery and handle components are covered under 1-year standard terms, extendable via service contracts. |
| 5. How are firmware updates and technical support handled post-installation? | Genoray delivers secure over-the-air (OTA) firmware updates quarterly, enhancing image processing algorithms, connectivity protocols, and compliance with evolving regulatory standards (e.g., IEC 60601-2-54). All registered units receive priority access to 24/7 technical support via phone, email, and live chat. Distributors are granted access to the Genoray Partner Portal for case tracking, diagnostic logs, and remote troubleshooting tools. |
Need a Quote for Genoray Portable X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


