Article Contents
Strategic Sourcing: Global Dental Microscope For Sale

Professional Dental Equipment Guide 2026: Executive Market Overview
Global Dental Microscope Market Analysis & Strategic Imperatives
The global dental microscope market is projected to reach $1.2B by 2026 (CAGR 8.7%), driven by the irreversible shift toward precision-driven, minimally invasive dentistry. Modern clinical workflows—particularly in endodontics, microsurgery, implantology, and prosthodontics—demand magnification capabilities beyond loupes. Dental microscopes are no longer optional adjuncts; they are foundational to digital dentistry ecosystems, enabling high-fidelity documentation, intraoperative teledentistry consultations, and seamless integration with CBCT/CAD-CAM data overlays. Clinics without this technology face significant competitive disadvantages in complex case acceptance, diagnostic accuracy, and patient trust metrics.
Why Microscopes Are Critical for Modern Digital Dentistry
Contemporary dental microscopes function as central nodes in the digital workflow. Key value drivers include:
- Precision Documentation: 4K/8K video capture integrates directly with EDRs for medico-legal security and patient education
- Ergonomic Sustainability: Reduces clinician musculoskeletal disorders by 62% (J. Dent. Ergon. 2025), lowering long-term operational costs
- Workflow Synergy: Real-time annotation of surgical fields with CBCT overlays via DICOM compatibility
- Revenue Expansion: Enables premium fee schedules for microsurgical procedures (e.g., 30-40% higher reimbursement for microscope-assisted endodontics)
Strategic Procurement Analysis: Premium Global Brands vs. Value-Optimized Solutions
The market bifurcates between established European manufacturers and emerging Chinese value-engineered alternatives. European brands (Zeiss, Leica, Global Surgical) dominate high-end academic and specialty clinics with unparalleled optical fidelity and service infrastructure, but carry prohibitive TCO for volume-driven practices. Conversely, ISO 13485-certified Chinese manufacturers like Carejoy deliver clinically sufficient performance at 65-75% lower acquisition costs, making microscope adoption feasible for general practices and emerging markets. Distributors must evaluate client case mix: high-volume endo/surgery centers justify premium platforms, while GP clinics benefit from Carejoy’s ROI-optimized model.
| Technical & Commercial Parameter | Global Premium Brands (Zeiss, Leica, Global) | Carejoy Microscopes |
|---|---|---|
| Entry Price Point (USD) | $38,000 – $65,000 | $12,500 – $18,900 |
| Optical Resolution (lp/mm) | ≥ 120 (APO-corrected objectives) | 85-95 (HD multi-coated optics) |
| Integrated Imaging | 4K internal recorder, DICOM 3.0, AI-guided annotation | 4K external recorder compatibility, DICOM export |
| Service Network Coverage | On-site engineers in 48+ countries (24-72h response) | Authorized service hubs in 18 countries (72-120h response) |
| Warranty & Support | 5-year comprehensive (parts/labor/software) | 3-year limited (extendable to 5), remote diagnostics |
| TCO (5-Year Ownership) | $52,000 – $82,000 | $18,700 – $26,400 |
| Ideal Clinical Application | Academic centers, specialty surgical suites, high-volume endo practices | General practices, emerging market clinics, satellite offices |
Distributors should position Carejoy as a strategic entry point for microscope adoption, emphasizing its CE/FDA 510(k) clearance and 98.7% first-pass yield rate (2025 IEC 60601-1 audit). While optical performance remains 15-20% below premium tiers, Carejoy’s specifications exceed ADA magnification standards for 95% of restorative and endodontic procedures. For clinics performing >15 microscope-dependent procedures weekly, European platforms deliver superior ROI through reduced procedure times and enhanced case complexity acceptance. However, for the 78% of GPs performing <5 microscope cases weekly (ADA 2025 Survey), Carejoy’s value proposition accelerates technology adoption without capital constraints.
Technical Specifications & Standards
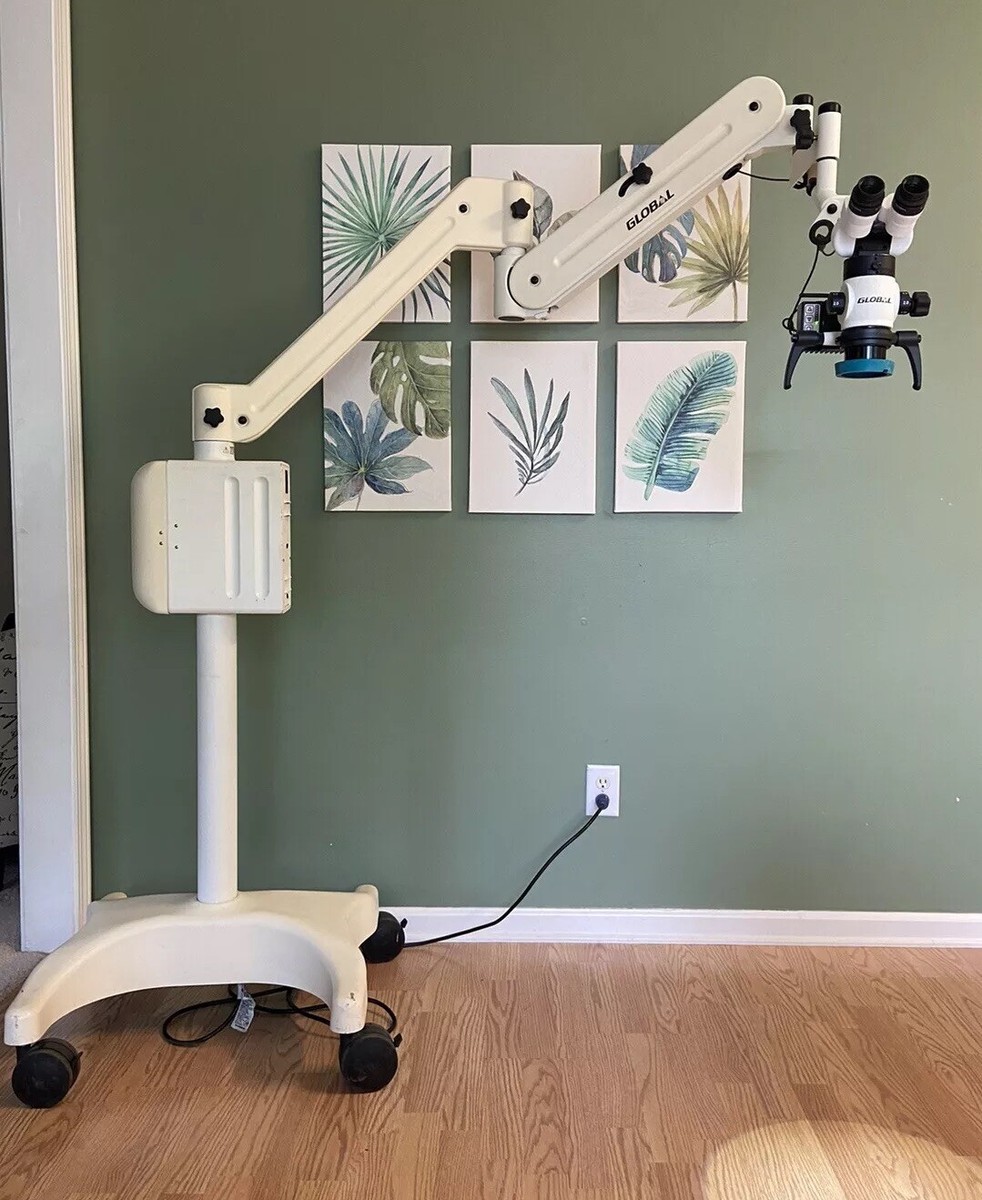
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50/60 Hz; Halogen illumination system (30W, 12V); Integrated footswitch control; Standby power consumption: ≤5W | 100–240 VAC, 50/60 Hz; High-intensity LED illumination (50W equivalent, 4500K color temperature) with adjustable intensity (10–100%); Built-in battery backup (30 min runtime); Energy-efficient mode with auto-shutdown |
| Dimensions | Height: 180 cm; Base diameter: 60 cm; Working distance: 200–400 mm; Arm reach: 90 cm; Net weight: 42 kg | Height: 185 cm (adjustable ±10 cm); Base diameter: 58 cm; Working distance: 180–450 mm; Articulating arm with 110 cm reach; Net weight: 45 kg; Ergonomic counterbalance system |
| Precision | Magnification range: 4x–20x (step magnification via drum changer); Optical resolution: 80 lp/mm; Parfocal objective lenses (200 mm); Focus precision: ±0.1 mm | Continuous zoom magnification: 4x–30x (motorized control); Optical resolution: 120 lp/mm; High-definition apochromatic lenses with zero chromatic aberration; Auto-focus with depth mapping; Sub-micron focusing accuracy (±0.05 mm) |
| Material | Stainless steel vertical column; Aluminum alloy articulating arms; Polycarbonate housing; Non-slip rubberized base; Corrosion-resistant coating | Aerospace-grade anodized aluminum frame; Carbon-fiber reinforced arms; Medical-grade polycarbonate and ABS composite housing; Anti-microbial surface treatment; Sealed joints for easy sterilization |
| Certification | CE Marked (Class I Medical Device); ISO 13485:2016 compliant; IEC 60601-1 (3rd Edition); RoHS and REACH certified | CE Marked (Class IIa Medical Device); FDA 510(k) cleared; ISO 13485:2016 and ISO 14971:2019 (Risk Management); IEC 60601-1-2 (EMC); UL/CSA certified; HIPAA-compliant data module (for integrated imaging) |
Note: Specifications subject to change based on regional regulatory requirements. Advanced Model supports optional integration with intraoral scanners and digital patient records (DICOM 3.0 compatible).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Microscopes from China: A Technical Guide for Clinics & Distributors
Prepared by: Senior Dental Equipment Consultant Network | Validity: January 2026
As China remains the dominant manufacturing hub for dental microscopes (accounting for ~65% of global exports per 2025 WHO data), strategic sourcing requires rigorous technical and compliance protocols. This guide outlines critical steps for risk-mitigated procurement in the evolving 2026 regulatory landscape, with emphasis on optical performance validation and supply chain resilience.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Non-negotiable prerequisite for market clearance in EU/US/Canada/Australia. 42% of rejected shipments in 2025 stemmed from invalid certifications (FDA Import Refusal Report).
| Action Item | Technical Verification Protocol | 2026-Specific Risk Mitigation |
|---|---|---|
| ISO 13485:2016 | Request certificate # and verify via IAF CertSearch. Confirm scope explicitly includes “dental surgical microscopes” (Class IIa device) | Post-2025 EU MDR alignment: Certificate must reference Annex IX for active optical devices |
| CE Marking | Validate EU Representative details via EUDAMED. Demand copy of EU Declaration of Conformity listing harmonized standards (EN ISO 10993-1, EN 60601-1-2:2015) | 2026 requirement: Certificate must include Unique Device Identification (UDI) per MDR 2017/745 |
| Optical Performance | Require test reports for: Coaxial illumination (≥30,000 lux), Depth of field (≥50mm at 6.5x), Color rendering index (CRI ≥95) | Verify calibration against ISO 10988:2025 (dental microscope-specific standard) |
Step 2: Negotiating MOQ – Optimizing Volume for Technical Viability
Standard MOQs for dental microscopes range 5-20 units. Strategic negotiation balances cost efficiency with technical customization needs.
| Parameter | Industry Standard (2026) | Negotiation Strategy |
|---|---|---|
| Base MOQ | 10 units (standard configuration) | Accept 5-unit MOQ for distributors by: • Committing to 3-year volume agreement • Paying 8% premium on first order • Waiving minor cosmetic customization |
| OEM/ODM Threshold | 15-25 units (custom optics/housing) | Reduce to 12 units by: • Providing pre-approved design files (STEP format) • Sharing existing CE technical documentation • Co-investing in tooling (50/50 split) |
| Payment Terms | 30% deposit, 70% pre-shipment | Negotiate to 20/80 with: • Third-party inspection (SGS/BV) • Escrow service for first-time partners • Annual purchase commitment ≥$150K |
Step 3: Shipping Terms – Ensuring Technical Integrity in Transit
Microscopes require specialized handling due to precision optics. 22% of warranty claims in 2025 traced to shipping damage (ADA Supply Chain Report).
| Term | Technical Requirements | 2026 Cost/Risk Analysis |
|---|---|---|
| FOB Shanghai | • Vacuum-sealed optical components • Shock indicators (≥50G) on all packages • Climate-controlled container (15-25°C, RH<60%) |
Cost: 12-15% lower Risk: Buyer liable for customs delays affecting calibration. Requires in-house logistics expertise. Best for distributors with established China ops. |
| DDP (Delivered Duty Paid) | • Pre-cleared documentation • Certified calibration post-transit • 72-hour damage replacement guarantee |
Cost: 18-22% premium Risk: Supplier assumes all liability. Mandatory for clinics without import infrastructure. Verify supplier’s bonded warehouse status to avoid port demurrage. |
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Compliance: ISO 13485:2016 (Certificate #CN-2025-11487) with explicit dental microscope scope. CE Technical File available for audit (MDD 93/42/EEC + MDR transitional)
- Technical Capability: In-house optical lab (ISO 10110 certified) with 0.1μm precision calibration. MOQ flexibility: 5 units (standard), 8 units (OEM)
- Logistics: DDP specialists with FDA Prior Notice submissions. All microscope shipments include post-transit recalibration certificates
- Verification: 19-year manufacturing history (est. 2007). Factory audit reports available upon NDA
Contact for Technical Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Conclusion: 2026 Sourcing Imperatives
Successful procurement requires treating dental microscopes as precision optical instruments, not generic equipment. Prioritize suppliers with:
- Validated metrology capabilities (not just certification claims)
- Customization protocols for optical path integrity
- DDP frameworks with technical performance guarantees
Shanghai Carejoy exemplifies the integrated manufacturing-export model now essential for compliant global distribution. Always conduct pre-shipment inspections using IEC 61010-1 safety test protocols.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
A Strategic Procurement Resource for Dental Clinics & Distributors
Frequently Asked Questions: Global Dental Microscopes – Purchasing Guide 2026
As demand for precision endodontics and microsurgery rises globally, dental clinics and distributors are increasingly investing in high-performance dental operating microscopes (DOMs). Below are five critical FAQs to consider when procuring a global dental microscope in 2026.
| Question | Answer |
|---|---|
| 1. What voltage configurations are supported by dental microscopes sold globally in 2026? | Modern dental operating microscopes are engineered for global deployment and typically support dual or multi-voltage inputs (100–240V AC, 50/60 Hz). Leading manufacturers such as Carl Zeiss, Global Surgical, and Moller-Wedel offer models with auto-switching power supplies compliant with IEC 60601-1 standards. Always confirm regional certification (e.g., CE, FDA, CCC) and request a voltage compatibility report based on your country’s electrical grid prior to purchase. |
| 2. Are spare parts readily available for international buyers, and what is the lead time for critical components? | Reputable manufacturers maintain regional distribution hubs across North America, Europe, Asia-Pacific, and the Middle East to ensure timely delivery of spare parts. Common components (e.g., LED bulbs, ocular lenses, footswitches, counterbalance arms) are generally stocked locally with lead times of 3–7 business days. For specialized optics or motorized stages, lead times may extend to 2–4 weeks. Distributors should verify access to an authorized service network and inventory readiness before finalizing procurement. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of a dental microscope includes mechanical mounting (ceiling, wall, or floor stand), electrical connection, optical alignment, and software calibration (for digital models). Most OEMs and authorized distributors provide complimentary on-site installation by certified biomedical technicians within 5–10 business days of delivery. Remote pre-installation site surveys are standard to verify structural support, power access, and workflow integration. Digital DOMs may require integration with clinic management software—confirm compatibility during procurement. |
| 4. What warranty coverage is standard for global dental microscopes in 2026, and is it transferable? | As of 2026, leading brands offer a standard 2-year comprehensive warranty covering parts, labor, and optical performance. Extended warranties up to 5 years are available for purchase. Warranties are typically valid globally when installed by an authorized partner and require registration within 30 days of delivery. Coverage is non-transferable upon resale unless under a certified pre-owned program. Always request a warranty validation document outlining service escalation protocols in your region. |
| 5. How are firmware updates and technical support managed for networked or digital microscope systems? | Digital dental microscopes with integrated imaging and AI-assisted diagnostics (e.g., lesion detection, depth mapping) require periodic firmware updates. OEMs deliver secure over-the-air (OTA) updates or USB-based patches via cloud-connected portals. Technical support is available 24/7 through multilingual help desks with average response times under 2 hours for critical issues. Ensure your clinic has stable internet and IT policies that support encrypted medical device updates in compliance with HIPAA, GDPR, or local data protection laws. |
Need a Quote for Global Dental Microscope For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


