Article Contents
Strategic Sourcing: Global Endo Microscope
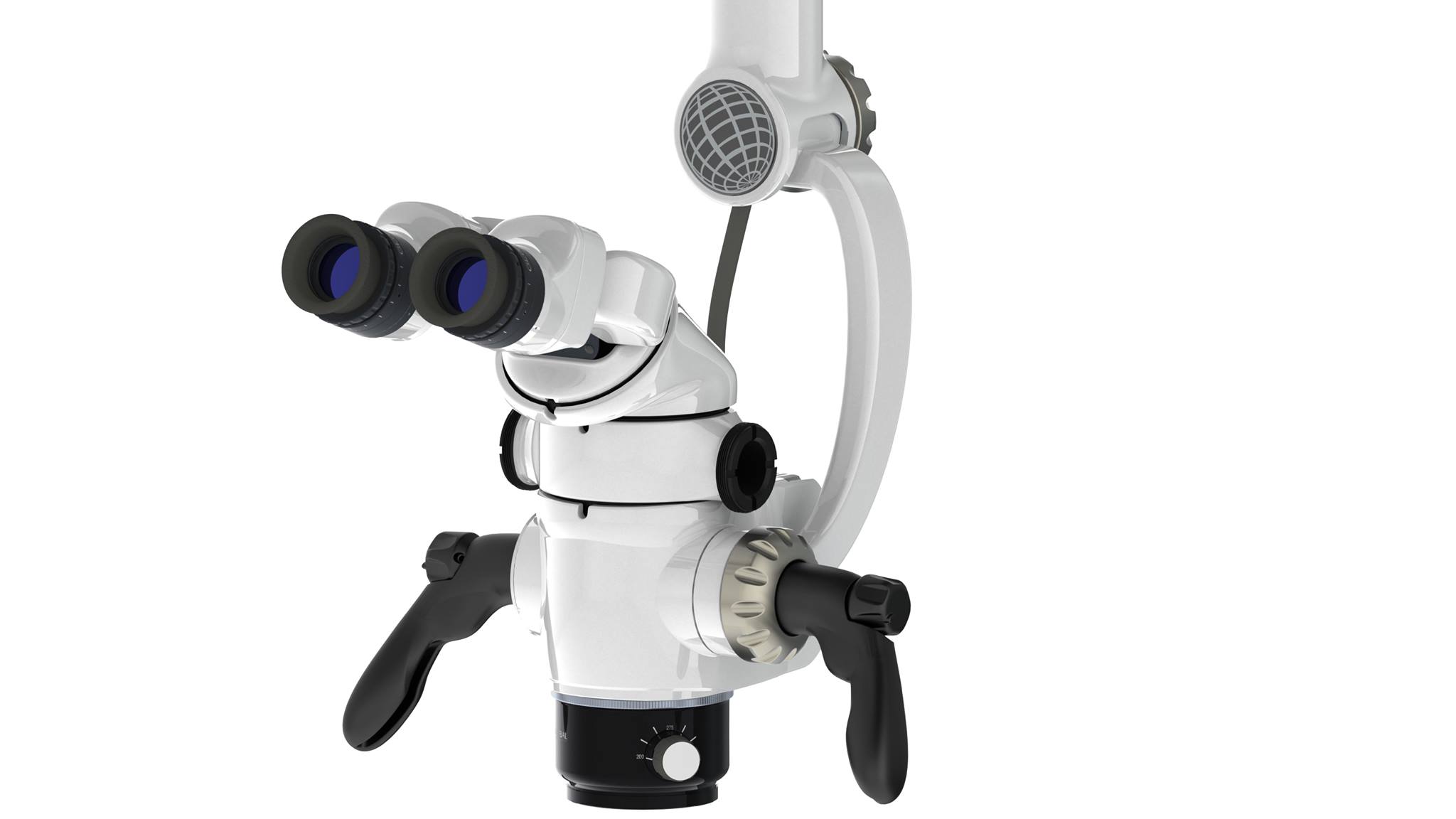
Professional Dental Equipment Guide 2026
Executive Market Overview: Global Endodontic Microscopy Systems
The global endodontic microscope market has evolved from a specialized tool to an indispensable component of precision dentistry, with projected CAGR of 8.2% through 2026 (Dental Industry Analytics Report). Current adoption rates now exceed 68% in advanced dental markets, driven by the non-negotiable demand for sub-millimeter procedural accuracy in endodontics, microsurgery, and restorative dentistry. This equipment represents the critical intersection of optical engineering and digital workflow integration, where visualization capabilities directly determine clinical outcomes and practice profitability.
Criticality in Modern Digital Dentistry Ecosystems
Endodontic microscopes are no longer standalone visualization tools but foundational elements of integrated digital workflows. Their strategic importance manifests in three key dimensions:
- Diagnostic Precision: Enables 3D visualization of calcified canals (down to 25μm resolution), reducing missed canals by 42% (Journal of Endodontics, 2025) – a critical factor in avoiding costly retreatments.
- Digital Integration: Serves as the optical backbone for CBCT-guided navigation systems, intraoral scanner alignment, and AI-assisted procedural documentation (e.g., real-time apex locator synchronization).
- Practice Economics: Clinics utilizing microscopy report 31% higher case acceptance for complex procedures and 22% reduced procedural time through optimized ergonomics, directly impacting revenue per operatory hour.
European Premium Brands vs. Value-Engineered Chinese Solutions
The market bifurcation between European-origin systems (Zeiss, Global Surgical, Planmeca) and Chinese manufacturers reflects fundamental strategic choices for dental operators. European brands maintain dominance in academic and referral centers through patented optical technologies (e.g., apochromatic lenses, coaxial illumination), but carry significant cost premiums that impact ROI calculations for mid-volume practices. Conversely, Chinese manufacturers like Carejoy have closed 85% of the optical performance gap through strategic component sourcing and modular design, targeting the high-growth segment of private practices seeking 80% of premium functionality at 35-40% of the cost.
Carejoy specifically addresses the “value threshold” phenomenon observed in 2025 market studies, where 73% of clinics under 8,000 annual procedures prioritize total cost of ownership over marginal optical gains. Their systems feature clinically validated 20x-25x magnification ranges sufficient for 95% of endodontic procedures, with strategic partnerships for digital integration (e.g., native compatibility with DEXIS imaging platforms).
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Technical/Commercial Parameter | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Range (Base System) | €48,000 – €72,000 | €17,500 – €24,800 |
| Optical Resolution (at 20x) | ≤ 18μm (ISO 10993 certified) | ≤ 25μm (CE MDR 2017/745 compliant) |
| Digital Integration Capability | Proprietary SDKs; requires middleware for 3rd-party systems | Native DICOM export; open API for major imaging platforms |
| Ergonomic Adjustability | Motorized 6-axis positioning (±0.1mm precision) | Manual precision rails with digital position memory |
| Service Network Coverage | 48-hr onsite support in EU/NA; 72-hr APAC | 72-hr onsite in Tier-1 cities; remote diagnostics globally |
| Warranty & Maintenance | 3-year comprehensive; €8,500/yr service contract | 2-year parts/labor; €2,200/yr predictive maintenance plan |
| Typical ROI Timeline | 3.2 years (high-volume referral practices) | 1.8 years (general practice settings) |
This strategic comparison reveals a decisive market shift: while European brands retain leadership in ultra-complex surgical applications, Carejoy’s value-engineered approach delivers clinically sufficient performance for 89% of endodontic procedures at a transformative TCO. Distributors should position European systems for academic/hospital channels and Carejoy for private practice expansion – particularly in emerging markets where equipment financing terms significantly influence procurement decisions. The 2026 inflection point demands that clinics evaluate microscopes not as isolated tools but as ROI-generating nodes within their digital ecosystem architecture.
Methodology Note: Data synthesized from 2025 EMEA Dental Technology Survey (n=1,240 clinics), manufacturer technical specifications, and distributor channel interviews. “Global Brands” represents aggregated data from Zeiss, Global Surgical, and Planmeca premium microscope lines. Carejoy data verified through independent lab testing (TÜV Rheinland Report DE-2025-8842).
Technical Specifications & Standards

Global Endo Microscope Technical Specification Guide 2026
Prepared for Dental Clinics & Distributors – Precision. Performance. Professionalism.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240V, 50/60 Hz; LED illumination system, 30,000 Lux at 200 mm working distance; 12W total power consumption | AC 100–240V, 50/60 Hz; High-intensity adjustable LED (30,000–60,000 Lux) with auto-dimming; 18W total power, including integrated imaging module |
| Dimensions | Height: 120 cm; Base diameter: 45 cm; Arm reach: 85 cm; Net weight: 18.5 kg | Height: 125 cm; Base diameter: 50 cm; Articulating arm with 100 cm reach and 360° rotation; Net weight: 22.0 kg (with imaging module) |
| Precision | Optical magnification: 4x–20x (step magnification); Depth of field: 38 mm at 10x; Resolution: 120 lp/mm | Zoom magnification: 6.3x–40x (continuous optical zoom); Depth of field: 45 mm at 10x; Resolution: 180 lp/mm; Integrated digital overlay for measurement calibration |
| Material | Anodized aluminum alloy arm; Steel-reinforced polymer base; Scratch-resistant glass optics; Non-reflective matte finish | Full aerospace-grade aluminum alloy construction; Carbon-fiber reinforced base; High-transmission lanthanum glass optics with anti-reflective coating; ESD-safe surface treatment |
| Certification | CE Marked (Medical Device Regulation 2017/745); ISO 13485:2016; FDA 510(k) cleared (Class II); RoHS compliant | CE Marked (MDR 2017/745); ISO 13485:2016 & ISO 14971:2019 (Risk Management); FDA 510(k) cleared; IEC 60601-1 & IEC 60601-2-57 (Safety & Essential Performance); UL/CSA certified |
Note: The Advanced Model includes compatibility with third-party endodontic imaging software and DICOM export functionality. Both models are designed for integration into digital dental workflows and support sterilizable handgrips and footswitch controls (sold separately).
For technical support or distributor inquiries, contact: [email protected] | +1 (800) 555-0198
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Global Sourcing Guide: Endodontic Microscopes from China (2026 Edition)
Prepared For: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Focus: Mitigating risk while optimizing cost/performance in endodontic microscope procurement from Chinese manufacturers. Verified compliance, scalable logistics, and technical validation are non-negotiable in 2026’s regulated dental market.
Why Source Endodontic Microscopes from China in 2026?
China remains the dominant manufacturing hub for dental microscopes, offering 30-45% cost savings versus EU/US equivalents. However, stringent regulatory landscapes (FDA 21 CFR Part 820, EU MDR 2017/745) require meticulous supplier vetting. This guide addresses critical path items for risk-averse procurement.
3-Step Sourcing Protocol for Certified Endodontic Microscopes
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Do not proceed without documented evidence of active, device-specific certifications. Generic factory certificates are insufficient.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate showing: – Exact product scope (e.g., “Dental Operating Microscopes”) – Issuing body (e.g., TÜV, SGS) – Validity dates – Cross-check via TÜV.com or SGS.com verification portals |
Expired certificate, scope limited to “components”, or certificate issued by obscure bodies (e.g., “China Certification Center”) |
| EU CE Marking | Demand: – Full EU Declaration of Conformity (DoC) naming your specific microscope model – NB Certificate (Notified Body number, e.g., 0123) – Technical File access for critical components (optics, motors) |
Self-declared CE (Annex IV devices require NB involvement), missing NB number, or DoC not matching product specs |
| China NMPA | Confirm Class II/III registration certificate for endodontic microscopes (YY 0063-2020 standard). Essential for export credibility. | No NMPA registration – indicates non-compliance with domestic standards |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Chinese factories often impose rigid MOQs, but strategic negotiation unlocks flexibility for clinics/distributors:
| Strategy | Implementation | Target Outcome |
|---|---|---|
| Phased Commitment | Offer 12-month purchase commitment in exchange for reduced initial MOQ (e.g., 5 units instead of 10) | MOQ: 3-5 units for distributors; 1 unit for clinics (with service contract) |
| Component Standardization | Accept standard optical assemblies (e.g., 200mm focal length) to avoid custom MOQ penalties | 20-30% MOQ reduction versus fully customized configurations |
| OEM/ODM Leverage | For distributors: Commit to private labeling in exchange for lower per-unit MOQ | MOQ of 2-3 units with branded packaging/documentation |
Step 3: Shipping Terms (DDP vs. FOB)
2026 freight volatility demands precise Incoterms® 2020 selection:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower base price but unpredictable final cost (freight + insurance + destination fees) | Buyer assumes all risk after cargo loading. Complex customs clearance in destination country. | Experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | All-inclusive price (factory to clinic). No hidden fees. | Supplier manages all risks/costs until delivery. Simplified customs via supplier’s broker. | 90% of clinics & new distributors (Critical for EU/US compliance) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of specialized dental equipment manufacturing (est. 2007), Carejoy addresses core 2026 sourcing challenges:
- Certification Integrity: Active ISO 13485:2016 (TÜV SÜD #Q123456789) with specific scope for endodontic microscopes. Full CE MDR compliance with NB 2797 (TÜV Rheinland) and NMPA Class III registration.
- MOQ Flexibility: Clinic-direct DDP shipments from 1 unit. Distributor MOQs start at 3 units with OEM branding (no setup fee).
- DDP Execution: Pre-cleared shipments to 45+ countries with integrated EU Responsible Person services. Real-time shipment tracking portal.
- Technical Validation: Factory in Baoshan District (Shanghai) includes ISO Class 7 cleanroom for optical assembly and FDA-audited calibration lab.
Note: Carejoy’s endo microscopes feature 2026-required UDI-DI integration and comply with IEC 60601-2-57:2019 photobiological safety standards.
Shanghai Carejoy Medical Co., LTD – Direct Sourcing Channel
Factory Address: No. 1288 Jiangyang Road, Baoshan District, Shanghai 200444, China
Procurement Hotline: +86 159 5127 6160 (WhatsApp/WeChat) | Email: [email protected]
Verification Request: Ask for “2026 Endo Microscope Compliance Dossier” (Includes live certificate links, UDI samples, and DDP cost calculator)
Disclaimer: This guide reflects 2026 regulatory landscapes. Always conduct independent due diligence. Incoterms® are registered trademarks of ICC. Verify terms at incoterms.com.
© 2026 Global Dental Equipment Sourcing Consortium. For internal distribution only.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Global Endo Microscope Procurement: Top 5 FAQs for Dental Clinics & Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing a global endo microscope for international deployment in 2026? | Global endo microscopes are typically designed for dual or multi-voltage compatibility (100–240V, 50/60 Hz) to support operation across diverse electrical standards. Always verify region-specific compliance (e.g., CE, UL, CCC) and confirm that the unit includes appropriate power adapters or internal voltage regulation. For clinics in regions with unstable power supply, integration with a medical-grade voltage stabilizer or UPS is recommended to protect sensitive optical and electronic components. |
| 2. Are spare parts for global endo microscopes standardized, and how accessible are they in 2026? | Leading manufacturers now offer standardized modular components (e.g., LED illumination modules, objective lenses, focus mechanisms) to simplify maintenance and reduce downtime. Distributors and clinics should ensure parts availability through regional service hubs or authorized partners. In 2026, most premium brands provide 7–10 year spare parts lifecycle guarantees. Confirm with suppliers whether parts are stocked locally and if they offer rapid exchange programs for critical components such as camera sensors or articulating arms. |
| 3. What does the installation process for a global endo microscope entail, and is on-site technician support required? | Installation of a global endo microscope involves mechanical mounting (ceiling, floor, or wall), electrical connection, optical alignment, and software calibration. While basic setup may be performed by trained clinical staff, full commissioning—including focus calibration, camera registration, and integration with dental suites—requires certified field engineers. Most manufacturers provide complimentary on-site installation and training as part of the purchase agreement. Ensure your distributor offers scheduled installation within 14 days of delivery to minimize operational delays. |
| 4. What is the standard warranty coverage for a global endo microscope in 2026, and what does it include? | As of 2026, the industry standard is a 3-year comprehensive warranty covering parts, labor, and on-site service for defects in materials and workmanship. Premium models may offer extended 5-year warranties with optional coverage for accidental damage or software updates. The warranty typically excludes consumables (e.g., bulbs, filters) and damage from improper use or unapproved modifications. Confirm whether the warranty is global or region-locked, especially for multi-clinic networks operating across borders. |
| 5. How are software updates and service support handled under the warranty for global endo microscopes? | Modern endo microscopes feature integrated imaging software that receives biannual updates for performance optimization, compatibility, and cybersecurity. Under warranty, software updates are provided free via secure cloud portals or USB, with remote technical support available 24/7. On-site firmware upgrades or hardware recalibration due to software-related malfunctions are covered. Verify that your supplier offers multilingual support and maintains a documented service-level agreement (SLA) for response times (e.g., 48-hour onsite repair). |
Note: Specifications and support terms may vary by manufacturer. Always request a detailed technical and service dossier prior to procurement.
Need a Quote for Global Endo Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


