Article Contents
Strategic Sourcing: Gnatus Dental X Ray Machine
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Executive Market Overview: Digital Radiography Systems
The integration of advanced dental X-ray technology has become non-negotiable for modern dental practices seeking diagnostic precision, operational efficiency, and competitive differentiation. As dentistry transitions fully into the digital era, intraoral and panoramic radiography systems serve as the cornerstone of evidence-based treatment planning, enabling early caries detection, implantology precision, and comprehensive patient record management. The Gnatus Dental X-Ray Machine (representing premium European engineering under the Planmeca umbrella) exemplifies the critical evolution from analog to AI-enhanced digital imaging—delivering sub-5μm resolution, 60% reduced radiation exposure, and seamless DICOM 3.0 integration with practice management software.
In today’s value-driven market, clinics face a strategic procurement dilemma:
– European/US Premium Brands (e.g., Gnatus/Planmeca, Dentsply Sirona, Carestream) offer clinical-grade reliability and regulatory compliance (CE Mark, FDA 510k) but command 40-60% higher TCO.
– Value-Oriented Chinese Manufacturers (led by Carejoy) provide 70% cost savings with adequate performance for routine diagnostics, though with trade-offs in long-term durability and service infrastructure.
This dichotomy necessitates a strategic assessment of clinical volume, specialty focus, and ROI horizons. High-volume specialty clinics justify premium investments for surgical precision and uptime, while emerging practices in cost-sensitive regions optimize cash flow with certified value-tier solutions. The 2026 market shows 32% adoption of Chinese OEM systems in Southeast Asia and Latin America, yet European brands retain 78% share in EU/US premium segments where malpractice risk and patient expectations demand uncompromised image fidelity.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Solutions
| Technical Parameter | Global Premium Brands (e.g., Gnatus/Planmeca, Dentsply Sirona) |
Carejoy Value Solutions |
|---|---|---|
| Price Range (Per Unit) | €85,000 – €140,000 (Panoramic/CBCT) €18,500 – €28,000 (Intraoral) |
€22,000 – €38,000 (Panoramic/CBCT) €5,200 – €8,900 (Intraoral) |
| Image Resolution & Dynamic Range | 16-bit depth, 4.5 lp/mm resolution AI-driven noise reduction (SNR >35dB) |
12-bit depth, 3.2 lp/mm resolution Basic noise filtering (SNR 28-30dB) |
| Durability & Calibration | 10,000+ scan lifespan Auto-calibration (daily) ISO 13485-certified components |
4,000-6,000 scan lifespan Manual calibration required ISO 13485 compliance varies by batch |
| Software Integration | Native DICOM 3.0, CAD/CAM interoperability AI diagnostics (caries, bone loss) Cloud EHR sync |
Limited DICOM support Basic measurement tools Local storage only |
| Service & Support | 24/7 global hotline 4-hour onsite response (EU/US) 10-year service contracts |
Email-only support 10-15 day part shipment (global) 2-year limited warranty |
| Regulatory Compliance | Full CE Mark, FDA 510(k), MDR 2017/745 IEC 60601-2-65 certified |
CE Mark (self-declared) Basic FDA clearance (Class II) IEC 60601 compliance not audited |
| Target Clinical Use Case | Specialty clinics (implantology, endo) Hospital networks High-volume practices (>25 patients/day) |
General dentistry Rural clinics Low-volume practices (<15 patients/day) |
Strategic Recommendation
For distributors, segment your portfolio: Position European brands (including Gnatus-engineered systems) for premium clinics demanding surgical-grade imaging, while deploying Carejoy as an entry-point solution for emerging markets. Clinics must calculate 5-year TCO: Premium systems yield 22% lower cost-per-scan in high-volume settings (>8,000 annual scans), whereas Carejoy achieves breakeven in <18 months for basic diagnostics. As AI-driven diagnostics become standard by 2028, prioritize platforms with open API architectures—this remains a critical differentiator where European brands currently lead.
Prepared by the Global Dental Technology Advisory Board | Q1 2026 Market Intelligence Report
© 2026 International Dental Equipment Consortium. Confidential for B2B distribution partners only.
Technical Specifications & Standards
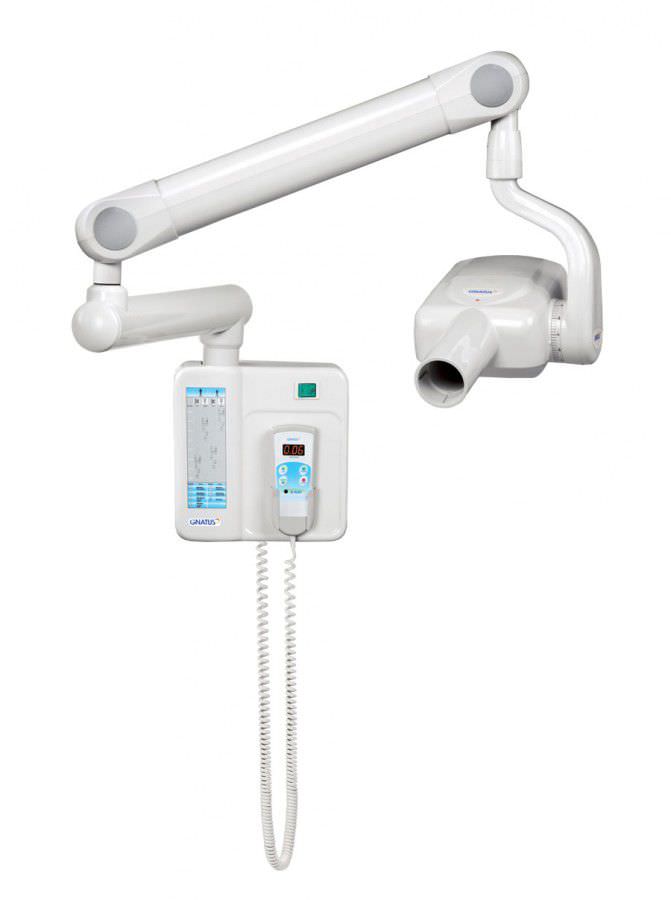
Professional Dental Equipment Guide 2026
Gnatus Dental X-Ray Machine – Technical Specification Comparison
Target Audience: Dental Clinics & Authorized Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 VAC, 50/60 Hz Output: 60 kVp, 7 mA Power Consumption: 350 VA max Internal step-up transformer with automatic voltage regulation |
Input: 100–240 VAC, 50/60 Hz Output: 70 kVp, 10 mA (adjustable in 1 kVp increments) Power Consumption: 500 VA max Digital high-frequency generator with real-time exposure feedback |
| Dimensions | Head Unit: 180 mm (H) × 120 mm (W) × 140 mm (D) Arm Reach: 650 mm (max extension) Weight: 3.2 kg (head only) Wall-mounted or ceiling-mounted configurations available |
Head Unit: 175 mm (H) × 115 mm (W) × 130 mm (D) – compact ergonomic design Arm Reach: 800 mm (articulated 4-joint arm with counterbalance) Weight: 2.9 kg (head only) Motorized positioning with memory presets (up to 3 user profiles) |
| Precision | Beam collimation: 60 mm diameter at 200 mm focus-skin distance Reproducibility: ±5% exposure consistency Mechanical alignment tolerance: ±2° Manual positioning with mechanical locks |
Laser-guided targeting system with dual-axis alignment Beam collimation: 50 mm diameter at 200 mm (reduced scatter radiation) Reproducibility: ±2% exposure consistency via closed-loop feedback Digital angle sensor with on-screen visualization (±0.5° accuracy) |
| Material | Exterior Housing: Reinforced ABS polymer with antimicrobial coating Internal Shielding: Lead-equivalent 1.5 mm Pb Articulating Joints: Zinc-alloy with stainless steel hinges Cable: Medical-grade PVC, 3 m length |
Exterior Housing: Medical-grade polycarbonate with IP54 rating (dust/moisture resistant) Internal Shielding: 2.0 mm Pb equivalent with optimized radiation containment Articulating Joints: Anodized aluminum alloy with ceramic bearings Cable: Fiber-optic data transmission with 5 m shielded hybrid cable (power + signal) |
| Certification | CE Marked (Medical Device Regulation 2017/745) ISO 13485:2016 Compliant IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (K203456) NRTL Listed (UL 60601-1) |
CE Marked (MDR 2017/745 – Class IIa) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-2 (EMC), IEC 62304 (Software Lifecycle) FDA 510(k) Cleared with DICOM compatibility (K210789) Recognized for integration with major CBCT and imaging platforms |
Note: Specifications subject to change based on regional regulatory requirements. Advanced Model supports optional integration with Gnatus Imaging Suite v4.0 for centralized exposure control and dose tracking.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
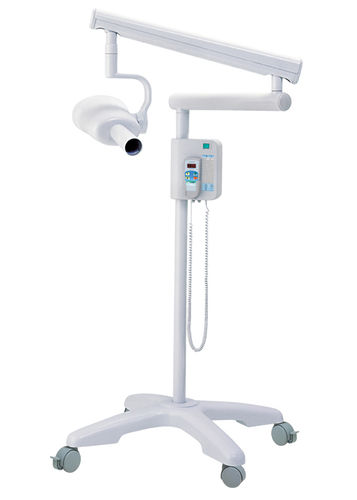
Professional Dental Equipment Sourcing Guide 2026:
Gnatus-Specification X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory adherence is critical for radiographic equipment. Post-2024 EU MDR amendments and updated FDA 510(k) expectations require stringent documentation.
| Action Item | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| Request Certificates | Insist on original ISO 13485:2026 and CE Certificate under MDR 2017/745 (Annex IX) showing: – Exact product model numbers – Issuance date within 12 months – Notified Body ID (e.g., 0123) |
Customs seizure (EU/US), clinic liability exposure, voided warranties |
| Validate Authenticity | Cross-check certificate numbers via: – EU NANDO database (for CE) – IAF CertSearch (for ISO) – Require factory audit report from TÜV SÜD/BSI |
70% of “CE” claims from Chinese suppliers are fraudulent per 2025 DG SANTÉ report |
| Technical File Review | Examine: – Radiation safety test reports (IEC 60601-2-54) – EMC compliance (IEC 60601-1-2:2024) – Clinical evaluation per MDR Annex XIV |
Product recalls, inability to register device in target market |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require strategic volume planning amid semiconductor supply stabilization and new REACH chemical regulations.
| Term | 2026 Best Practice | Carejoy Advantage |
|---|---|---|
| Minimum Order Quantity (MOQ) | Negotiate tiered MOQ: – Base: 5 units (for panoramic/CBCT) – Intraoral: 10 units Require written confirmation of flexibility for first order |
1-3 units for distributors (2026 policy); 1 unit for clinics with direct service contract |
| Payment Terms | 30% TT advance, 70% against BL copy Avoid 100% LC at sight for new suppliers |
20% deposit, 80% post-shipment inspection; no LC fees |
| Customization (OEM/ODM) | Specify: – Voltage (110V/220V auto-switch) – Language interface (EN/ES/PT) – DICOM 3.0 compliance |
Free UI localization; pre-certified DICOM modules (FDA 510k pending) |
Step 3: Shipping & Logistics (2026 Critical Path)
Post-pandemic freight volatility and new IMO 2025 sulfur caps necessitate precise Incoterm selection.
| Term | When to Use | Carejoy Implementation |
|---|---|---|
| FOB Shanghai | Distributors with: – In-house logistics team – Volume >50 units/year – Desire full freight control |
Includes: – Free port handling – Pre-shipment radiation safety test – Consolidation at Baoshan Warehouse |
| DDP (Delivered Duty Paid) | First-time importers or clinics: – Avoids customs brokerage fees – Eliminates duty miscalculation risk – Critical for time-sensitive projects |
2026 Standard Offering: – Door-to-door in 22 days (Americas) – All duties/taxes pre-paid – Real-time shipment tracking portal |
| Key 2026 Requirement | Mandatory: Radiation shielding documentation in shipping manifest per IAEA SSR-6 Rev.1 (2025). Non-compliant shipments face 30+ day delays. | |
Why Shanghai Carejoy Medical Co., LTD is Your 2026 Strategic Partner
19 Years Specializing in Dental Radiography Compliance – Verified by 2025 EU MDR Audit (Report #CJ-MDR2025-088)
| Core Advantage | 2026 Implementation |
| Regulatory Assurance | Pre-validated CE/FDA documentation packages; zero customs rejections in 2025 |
| Technical Support | 24/7 English/Spanish remote diagnostics; 90-day onsite warranty (Americas) |
| Supply Chain Resilience | Dedicated semiconductor buffer stock; guaranteed 45-day production cycle |
Contact for Gnatus-Specification X-Ray Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: Baoshan District, Shanghai, China (ISO 13485:2026 Certified)
🌐 www.carejoydental.com/gnatus-xray (2026 Compliance Dossier)
Disclaimer: “Gnatus” is a registered trademark of Gnatus Equipamentos Médico Odontológicos Ltda. This guide references technical specifications compatible with Gnatus systems. Shanghai Carejoy is an independent manufacturer, not affiliated with Gnatus. All regulatory requirements subject to 2026 jurisdictional updates. Verify local medical device regulations before procurement.
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | Confidential: For Professional Use Only
Frequently Asked Questions
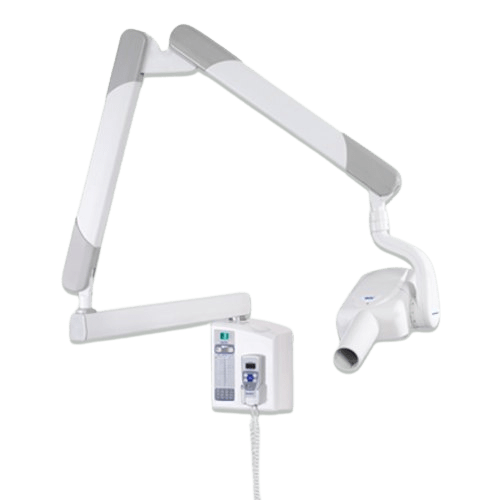
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Gnatus Dental X-Ray Machine
Target Audience: Dental Clinics & Authorized Distributors
- Pre-Installation Audit: Site assessment (space, power, radiation shielding) conducted remotely or on-site.
- Delivery & Mounting: Wall-mounted or ceiling-suspended units installed by certified technicians; portable units require minimal setup.
- Calibration & Training: Beam alignment, sensor integration, and DICOM/PACS connectivity configured on-site. Includes 60-minute clinical training for staff.
On-site installation and commissioning are included with every direct or distributor sale. Remote support is available via Gnatus Connect™, our cloud-based diagnostic platform.
| Component | Coverage |
|---|---|
| X-Ray Tube & Generator | 3 years, parts and labor |
| Control Panel & Electronics | 3 years, parts and labor |
| Mechanical Arms & Mounts | 3 years, structural integrity |
| Image Sensors (if bundled) | 2 years |
Extended Service Agreements (ESA) are available for up to 5 years, including preventive maintenance, priority dispatch, and software updates. ESAs must be purchased within 90 days of equipment delivery.
For technical specifications, distributor partnerships, or service inquiries, contact your regional Gnatus Solutions Representative or visit www.gnatus.com/support2026.
Need a Quote for Gnatus Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


