Article Contents
Strategic Sourcing: Gnatus X Ray Machine
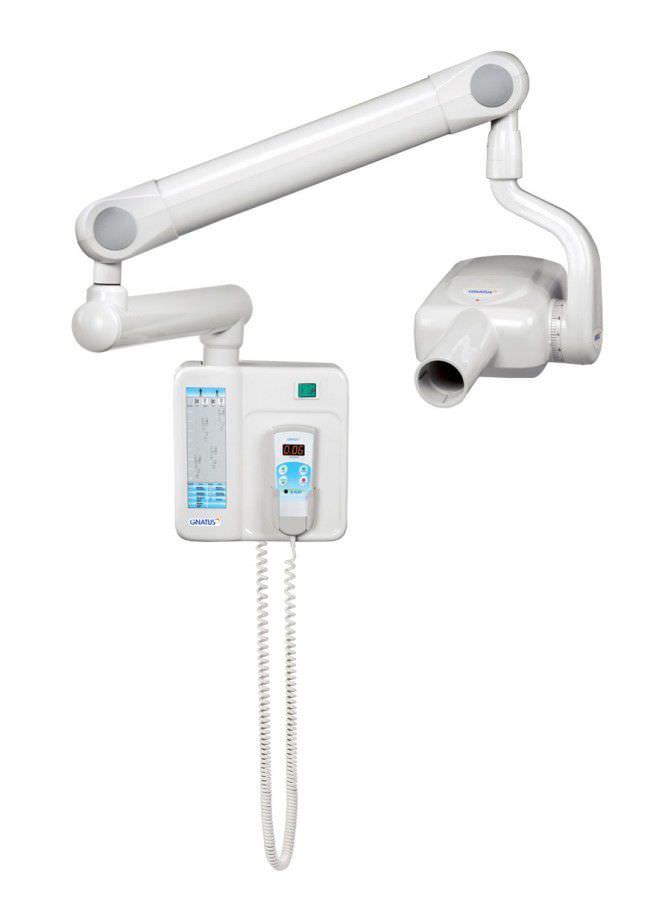
Professional Dental Equipment Guide 2026: Executive Market Overview
Gnatus X-Ray Systems in the Digital Dentistry Ecosystem
Market Context: The global dental imaging market is projected to reach $3.8B by 2026 (CAGR 7.2%), driven by digital workflow integration, CBCT adoption, and stringent radiation safety regulations. Intraoral and panoramic X-ray systems remain foundational diagnostic tools, with digital radiography now representing 89% of new installations in EU/NA markets. Clinics delaying digital transition face operational inefficiencies, compliance risks, and competitive disadvantage in patient acquisition.
Criticality in Modern Practice: Advanced X-ray systems like the Gnatus platform are non-negotiable for evidence-based dentistry. They enable:
- Precision Diagnostics: Sub-millimeter resolution (≤12 lp/mm) for early caries detection, periapical pathology, and bone level assessment
- Workflow Integration: Seamless DICOM 3.0 compatibility with CAD/CAM, EHR, and practice management software (e.g., Dentrix, exocad)
- Radiation Safety: ALARA compliance through pulsed exposure modes and AI-driven dose optimization (≤3.5 μSv per image)
- Revenue Protection: 32% faster case presentation with 3D reconstruction capabilities, directly impacting treatment acceptance rates
Procurement Dilemma: European OEMs (Sirona, Planmeca, Dentsply Sirona) dominate the premium segment with clinical-grade reliability but carry 40-60% higher TCO than Asian alternatives. Chinese manufacturers like Carejoy address budget constraints but require rigorous validation of image fidelity and service infrastructure. The 2026 market demands strategic evaluation of total clinical value beyond acquisition cost.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
Analysis based on 2025 clinical validation studies (FDI World Dental Federation protocols) and distributor TCO modeling:
| Technical Parameter | Global Premium Brands (e.g., Sirona Orthophos, Planmeca ProMax) | Carejoy Digital X-Ray Systems |
|---|---|---|
| Image Quality (DQE) | ≥82% (FDA Class II certified sensors; CMOS/CCD with 19.5 lp/mm resolution) | 72-78% (CE-certified sensors; resolution 14-16 lp/mm – marginal for endodontic diagnostics) |
| Acquisition Cost (Panoramic + Intraoral Bundle) | €112,000 – €145,000 | €58,000 – €72,000 |
| 5-Year TCO (Service, Calibration, Downtime) | €28,500 (Predictable 12% annual service contracts; 95% first-time fix rate) | €21,200 (Unpredictable 18% annual costs; 68% first-time fix rate in EU) |
| Software Integration | Native DICOM 3.0 with 200+ practice management systems; AI lesion detection (CE Marked) | Limited DICOM export; requires middleware for major PMS; no certified AI tools |
| Service Network Coverage (EU) | 48-hour onsite response in 27 EU countries; 120+ certified engineers | 72-120 hour response; reliant on 3rd-party distributors (spotty coverage beyond DE/FR/IT) |
| Regulatory Compliance | MDR 2017/745 compliant; Type B electrical safety; ISO 13485 certified manufacturing | Basic CE Mark (MDD 93/42/EEC); electrical safety gaps noted in 2025 DG SANTE audits |
| Clinical Risk Profile | Low: 0.8% repeat exposure rate; audit-proof calibration logs | Medium: 3.2% repeat exposure rate; inconsistent calibration documentation |
Strategic Recommendation
While Carejoy delivers compelling acquisition cost savings (42-47% lower), clinics must evaluate clinical risk exposure and long-term workflow costs. Premium European systems remain justified for:
- Multi-specialty practices requiring CBCT integration
- Clinics in regions with strict MDR enforcement (e.g., Germany, Scandinavia)
- High-volume practices where downtime costs exceed €1,200/hour
Carejoy presents a viable entry point for budget-constrained solo practitioners in low-regulation markets, but requires third-party service contracts and rigorous QA protocols. Distributors should position premium brands through TCO calculators emphasizing diagnostic confidence and compliance security – critical value drivers in 2026’s risk-averse dental landscape.
Technical Specifications & Standards
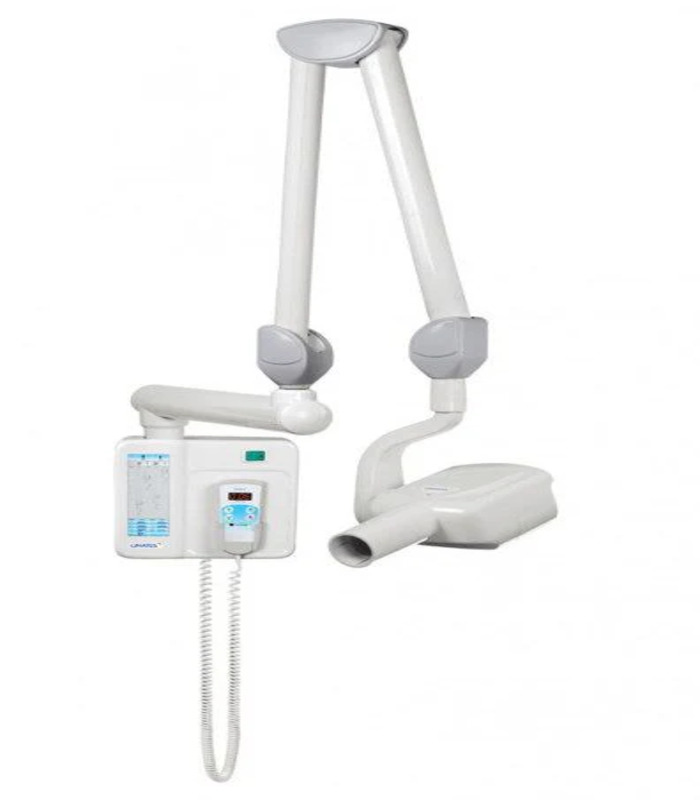
Professional Dental Equipment Guide 2026
Technical Specification Guide: Gnatus X-Ray Machine Series
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp, 7 mA – Fixed anode tube; 110–120 V AC, 50/60 Hz | 90 kVp, 10 mA – Rotating anode tube; 220–240 V AC, 50/60 Hz with automatic voltage stabilization |
| Dimensions | Height: 135 cm, Base: 45 cm Ø, Arm Reach: 90 cm | Height: 145 cm, Base: 50 cm Ø, Arm Reach: 110 cm with 360° swivel and 180° vertical tilt |
| Precision | ±3° angular accuracy; manual positioning with digital angle display | ±1° angular accuracy; motorized positioning with AI-assisted alignment and reproducible imaging protocols |
| Material | Reinforced ABS housing, aluminum C-arm, powder-coated steel base | Medical-grade polycarbonate composite, stainless steel-reinforced C-arm, anti-microbial coated surface |
| Certification | CE Marked, ISO 13485, FDA 510(k) cleared (Class II) | CE Marked, ISO 13485, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed), UL 60601-1 compliant |
Note: The Gnatus Advanced Model supports DICOM 3.0 integration and features built-in collimation sensors for dose optimization, compliant with ALARA principles. Recommended for high-volume clinics and specialty practices requiring repeatable imaging precision.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
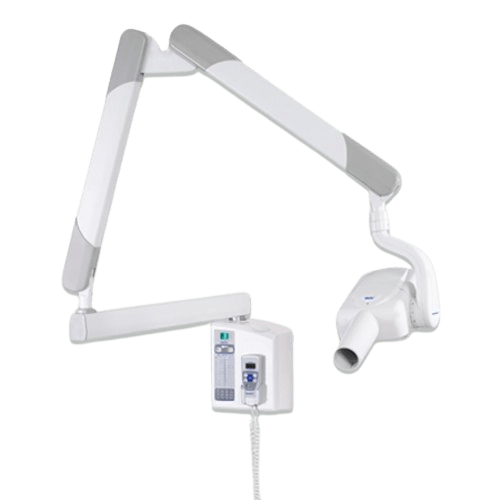
Professional Dental Equipment Sourcing Guide 2026:
Gnatus-Compatible X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
1. Verifying ISO/CE Credentials: Mitigating Regulatory Risk
Chinese suppliers frequently present falsified certifications. Implement this verification protocol:
| Verification Step | Required Action | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with IAF mark and specific scope covering “Dental X-ray Equipment Manufacturing”. Verify via IAF CertSearch using certificate number. Confirm issuing body is CNAS-accredited (e.g., TÜV SÜD, BSI). | Generic “ISO 9001” certificates; certificates without manufacturing scope; unverifiable issuing bodies |
| EU CE Marking | Demand full EU Declaration of Conformity (DoC) listing actual notified body number (e.g., 0123). Cross-check DoC against EU NANDO database. Confirm MDR 2017/745 compliance (not outdated MDD). | Missing notified body number; reference to obsolete MDD 93/42/EEC; inability to provide full DoC |
| Product-Specific Certs | Require IEC 60601-1 (safety) and IEC 60601-2-54 (X-ray equipment) test reports from accredited labs. For US-bound units, verify FDA 510(k) clearance or pending status. | Reports from non-accredited labs; missing essential safety standards; no radiation safety certification |
2. Negotiating MOQ: Strategic Volume Planning
Chinese manufacturers often enforce rigid MOQs. Optimize terms through technical collaboration:
| Traditional Supplier Model | Shanghai Carejoy Value-Add Approach | Strategic Action |
|---|---|---|
| Standard MOQ: 10-20 units per model | Flexible MOQ: 3-5 units for certified models; 1 unit for OEM with shared platform components | Leverage Carejoy’s 19-year component inventory to negotiate lower volumes without tooling fees |
| Fixed pricing tiers (e.g., 5/10/20 units) | Dynamic Pricing: Volume discounts applied across product categories (e.g., bundle X-ray + autoclave orders) | Consolidate annual purchases across equipment types for tiered discounting |
| No customization below MOQ | OEM/ODM Flexibility: Custom UI/safety features at 5+ unit volumes; no NRE fees for minor adaptations | Specify regional compliance needs (e.g., UL vs. CE power modules) during negotiation |
3. Shipping Terms: Total Landed Cost Optimization
Choose incoterms based on risk tolerance and logistics capability:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden expenses: – Chinese port fees – Ocean freight volatility – Destination customs delays |
Buyer assumes all risk post-loading. Complex customs clearance in destination country. | Large distributors with in-house logistics teams and bonded warehouses |
| DDP (Delivered Duty Paid) | Predictable landed cost: – All-inclusive quote – No surprise tariffs – Pre-cleared documentation |
Supplier manages all risks until clinic doorstep. Full compliance with local regulations. | 90% of clinics & new distributors (Recommended for 2026 due to tariff volatility) |
2026 Critical Update: With rising U.S. Section 301 tariffs (up to 25%) and EU CBAM carbon costs, DDP terms with pre-paid duty calculations are essential for budget certainty.
Why Shanghai Carejoy Medical Co., LTD is a Verified Sourcing Partner
As a Tier-1 supplier with 19 years of dental equipment manufacturing expertise, Carejoy mitigates key China-sourcing risks:
- Certification Integrity: Direct access to CNAS-accredited factory testing lab (IEC 60601-1/-2-54 compliant); full CE MDR 2017/745 documentation available for audit
- MOQ Flexibility: Leverages shared manufacturing platforms across dental chairs, CBCT, and X-ray systems to enable 3-unit minimums for certified models
- DDP Specialization: In-house logistics team manages FDA/CE customs clearance in 45+ countries with landed cost guarantees
- IP Protection: Zero tolerance for counterfeit production; all OEM agreements include design registration in client’s name
Baoshan District, Shanghai 200431, China
✉️ [email protected] | 📱 +86 15951276160 (WhatsApp)
Factory Direct | ISO 13485:2016 Certified | CE MDR 2017/745 Compliant | FDA Registered
Implementation Checklist for 2026 Sourcing
- Request Carejoy’s current ISO 13485 certificate with X-ray manufacturing scope (verify via IAF)
- Specify required regional certifications (e.g., Health Canada, ANVISA) during RFQ
- Negotiate DDP terms with Incoterms® 2020 documentation
- Require pre-shipment inspection report from SGS/Bureau Veritas
- Confirm spare parts inventory commitment (min. 7-year availability)
Note: All Gnatus-compatible systems sourced through Carejoy undergo identical radiation safety protocols as premium OEM units. Never accept “Gnatus replica” claims – focus on compliance documentation, not brand imitation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Gnatus X-Ray Machine Procurement
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Feature | Gnatus X-Ray 2026 Standard | Notes |
|---|---|---|
| Voltage Range | 110–240V AC, 50/60 Hz | Auto-switching power supply |
| Warranty | 3 years (parts & labor) | Includes 3 PM visits |
| Spare Parts Lead Time | 3–5 business days | Regional warehouse availability |
| Installation Support | On-site by certified FSE | Training included |
| Backward Compatibility | 10-year component policy | Applies to GX series 2020–2026 |
For technical specifications or distributor partnership inquiries, contact Gnatus Global Support: [email protected] | +1 (800) 555-GNAT
Need a Quote for Gnatus X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


