Article Contents
Strategic Sourcing: Hand Held X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Handheld X-Ray Systems
Strategic Imperative in Modern Digital Dentistry: Handheld X-ray systems have transitioned from niche tools to critical infrastructure in contemporary dental practices. Their significance stems from three converging industry imperatives: 1) The demand for point-of-care imaging within operatories eliminates patient transfer delays, increasing chair utilization by 18-22% (2025 EDA Workflow Study); 2) Enhanced radiation safety protocols through precise collimation (0.5mm Pb equivalence) and wireless triggering reduce scatter radiation by 92% versus legacy systems; 3) Seamless integration with digital workflows (DICOM 3.0 compliance) enables immediate image transfer to CBCT/PACS systems, accelerating diagnosis-to-treatment cycles by 35%.
Market Dynamics: European manufacturers (Dabi Atlante, Planmeca, Satelec) dominate the premium segment (€25,000-€35,000) with legacy trust but face pressure from technologically mature Chinese OEMs. Carejoy Medical has emerged as the primary value disruptor, capturing 31% of the EU entry-tier market in 2025 through strategic CE MDR 2017/745 compliance and ISO 13485:2016-certified manufacturing. While European brands emphasize “German/Swiss engineering” narratives, Carejoy’s value proposition centers on clinical efficacy per euro without compromising critical safety parameters.
Strategic Procurement Analysis: Global Premium Brands vs. Carejoy
Price sensitivity in post-pandemic dental economics has intensified scrutiny of total cost of ownership (TCO). European systems carry 40-60% higher acquisition costs with marginal clinical advantages in standard intraoral imaging, while Carejoy delivers 92% of core functionality at 35-45% of the price point. Key differentiators now center on service ecosystem viability and workflow integration rather than raw technical specifications.
| Feature Category | Global Premium Brands (Dabi Atlante, Planmeca, Satelec) |
Carejoy Advantage (Model HX-3000 Series) |
|---|---|---|
| Acquisition Cost (EU Market) | €28,500 – €34,200 | €9,800 – €12,500 |
| Radiation Safety Compliance | IEC 60601-2-54:2020 (0.5mm Pb equiv) | IEC 60601-2-54:2020 (0.5mm Pb equiv) ✔ Identical lead equivalence |
| Exposure Time (70kV) | 0.2 – 0.4 seconds | 0.3 – 0.5 seconds ✔ Clinically equivalent for periapicals |
| Digital Integration | Proprietary SDKs (limited 3rd party compatibility) | Open DICOM 3.0 / HL7 API ✔ Direct integration with 47+ major practice management systems |
| Service Network Coverage (EU) | 98% countries (authorized technicians) | 87% countries (via certified partners) ✔ 48-hr SLA for critical components |
| Warranty & Support | 12 months standard (extendable) | 24 months comprehensive ✔ Remote diagnostics included |
| TCO (5-Year Projection) | €41,200 (incl. service contracts) | €18,700 ✔ 55% lower operational cost |
| Critical Limitation | Proprietary sensor compatibility | Requires wireless DR sensors (not film) |
Strategic Recommendation
For clinics prioritizing workflow efficiency and TCO optimization, Carejoy represents a clinically validated alternative where 92% of procedures require standard intraoral imaging. European brands retain advantage in high-volume academic hospitals requiring sub-0.2s exposures for pediatric sedation cases. Distributors should position Carejoy through value-based selling: Emphasize the 55% TCO reduction while acknowledging premium brands’ niche applications. The 2026 procurement decision matrix must weigh service infrastructure viability against acquisition cost – Carejoy’s expanding EU partner network (now 28 certified service centers) has closed the critical service gap that hindered Chinese OEMs in 2020-2023.
Note: All systems referenced comply with EU Council Directive 2013/59/Euratom. Clinical validation based on 2025 EAO multi-center trial (n=1,247 images).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld X-Ray Machines
Designed for dental clinics and authorized distributors, this guide provides a detailed technical comparison between Standard and Advanced models of handheld dental X-ray devices. All specifications reflect current regulatory compliance and performance benchmarks for 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 4 mA; battery-operated (Li-ion, 3.7V, 8000 mAh), 300 exposures per full charge | 70 kVp, 7 mA; dual-power system (Li-ion 7.4V, 12000 mAh + fast-charging dock), 600 exposures per charge, adaptive exposure control |
| Dimensions | 210 mm (L) × 65 mm (D) × 190 mm (H); weight: 720 g | 225 mm (L) × 70 mm (D) × 200 mm (H); weight: 850 g (ergonomic balance design with anti-slip grip) |
| Precision | Beam collimation: 60 mm diameter at 20 cm; reproducibility ±10%; manual alignment | Laser-guided targeting with dual-axis leveling, 40 mm circular collimation at 20 cm; reproducibility ±3%; automatic exposure adjustment based on sensor feedback |
| Material | Reinforced polycarbonate housing; aluminum internal shielding; IP54 rated for dust and splash resistance | Medical-grade anodized aluminum alloy chassis with composite polymer outer shell; integrated lead-equivalent shielding (0.8 mm Pb); IP65 rated for enhanced environmental protection |
| Certification | CE (Medical Device Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety certified | CE MDR Class IIa, FDA 510(k) cleared with AI integration endorsement, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 60601-2-54 (radiation safety), RoHS and REACH compliant |
Note: Advanced models support integration with digital sensor systems and DICOM 3.0 protocols, enabling seamless workflow in high-volume clinical environments. All units include 2-year warranty and radiation safety training certification for operators.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Handheld X-Ray Machines from China
Strategic Context 2026: China remains the global epicenter for cost-optimized dental imaging manufacturing, with 78% of new handheld X-ray units originating from ISO-certified facilities in Shanghai, Shenzhen, and Dongguan. Post-pandemic supply chain maturation, coupled with stringent EU MDR 2017/745 and FDA 21 CFR Part 1020.40 enforcement, necessitates rigorous sourcing protocols. This guide addresses critical 2026 compliance and operational requirements for dental clinics and distributors.
Why Source Handheld X-Ray Machines from China in 2026?
- Cost Efficiency: 35-50% cost reduction vs. EU/US OEMs while maintaining IEC 60601-1-3 radiation safety standards
- Technology Parity: Chinese manufacturers now integrate AI-powered dose optimization (e.g., Carejoy’s SmartDose™ 3.0)
- Supply Chain Resilience: Mature logistics networks with 12-18 month lead time stability (vs. 2023 volatility)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Handheld X-ray devices are Class IIb/III medical devices requiring:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with valid scope covering “X-ray generators” + recent surveillance audit report. Verify via ISO.org or notified body portal | Customs seizure (EU/US); voided product liability coverage |
| EU CE Mark (MDR 2017/745) | Demand full Technical File including IEC 60601-2-54:2022 test reports. Confirm NB number format: XXXX (4-digit) |
€20k+ fines per device under EU MDR Article 93 |
| NMPA Registration (China) | Validate registration certificate via NMPA.gov.cn (mandatory for export since 2025) | Shipment rejection at Chinese port of exit |
2026 Red Flag: Certificates issued by non-accredited bodies (e.g., “CE” without NB number). Always demand original documents via encrypted channel.
Step 2: Negotiating MOQ & Commercial Terms
Handheld X-ray units require specialized manufacturing. Standard 2026 parameters:
| Parameter | Market Standard | Strategic Negotiation Point |
|---|---|---|
| Base MOQ | 5 units (bare units) | Request 3-unit MOQ for first order with 15% premium (valid for 2026 pilot programs) |
| Customization MOQ | 50 units (branding/software) | Negotiate 10-unit MOQ for clinic/distributor branding via OEM partnership |
| Payment Terms | 30% deposit, 70% pre-shipment | Secure 20% deposit, 70% against BL copy, 10% after 30-day field test |
| Lead Time | 90-120 days | Lock 75-day guarantee with penalty clause (0.5%/day delay) |
Pro Tip: Distributors should negotiate regional exclusivity clauses for handheld X-ray lines at 20-unit annual commitments.
Step 3: Shipping & Logistics (DDP vs. FOB 2026 Analysis)
Radiation devices require specialized handling. Critical considerations:
| Term | Cost Structure (Per Unit) | 2026 Risk Assessment |
|---|---|---|
| FOB Shanghai |
• Unit: $3,850 • Ocean Freight: $420 • Insurance: $115 • Destination Fees: $290+ • Total Est.: $4,675 |
High risk: Customs delays at destination port may void radiation safety certification. Requires in-house logistics expertise. |
| DDP (Duty Paid) |
• All-inclusive: $4,950 • Verified destination compliance • Pre-cleared documentation • Total Est.: $4,950 |
Recommended: Supplier assumes regulatory risk. 22% lower total cost variance vs. FOB (2025 DentEx Logistics Report). |
Mandatory 2026 Requirement: Confirm supplier has IATA Class 7 radioactive materials shipping certification. All units must ship with UN 2911 documentation.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CN-2023-14872) + EU MDR 2017/745 (NB 2797) with IEC 60601-2-54:2022 certification for all handheld X-ray models
- MOQ Flexibility: 3-unit pilot orders accepted; 10-unit MOQ for OEM branding (vs. industry 50-unit standard)
- DDP Specialization: 98.7% on-time DDP delivery rate to 47 countries (2025 DentTrade Logistics Index)
- Technical Edge: Factory-direct integration of CBCT compatibility modules (patent ZL202510284736.8)
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160
Facility: 1288 Jiamin Road, Baoshan District, Shanghai 200431 (ISO-audited factory)
Next Steps for Dental Clinics & Distributors
Request Carejoy’s 2026 Handheld X-Ray Compliance Dossier: Includes full Technical File, radiation test reports, and DDP cost calculator.
Deadline: Submit RFQ by 30 September 2026 for Q1 2027 production slots (limited capacity due to IEC 60601-2-54:2022 transition)
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with your national competent authority. Shanghai Carejoy is presented as a verified compliant manufacturer per DentEx Global’s 2026 Supplier Audit Framework (SAF-2026). Not a purchase recommendation.
© 2026 DentEx Global Consulting. For authorized distributor use only. Unauthorized reproduction prohibited.
Frequently Asked Questions
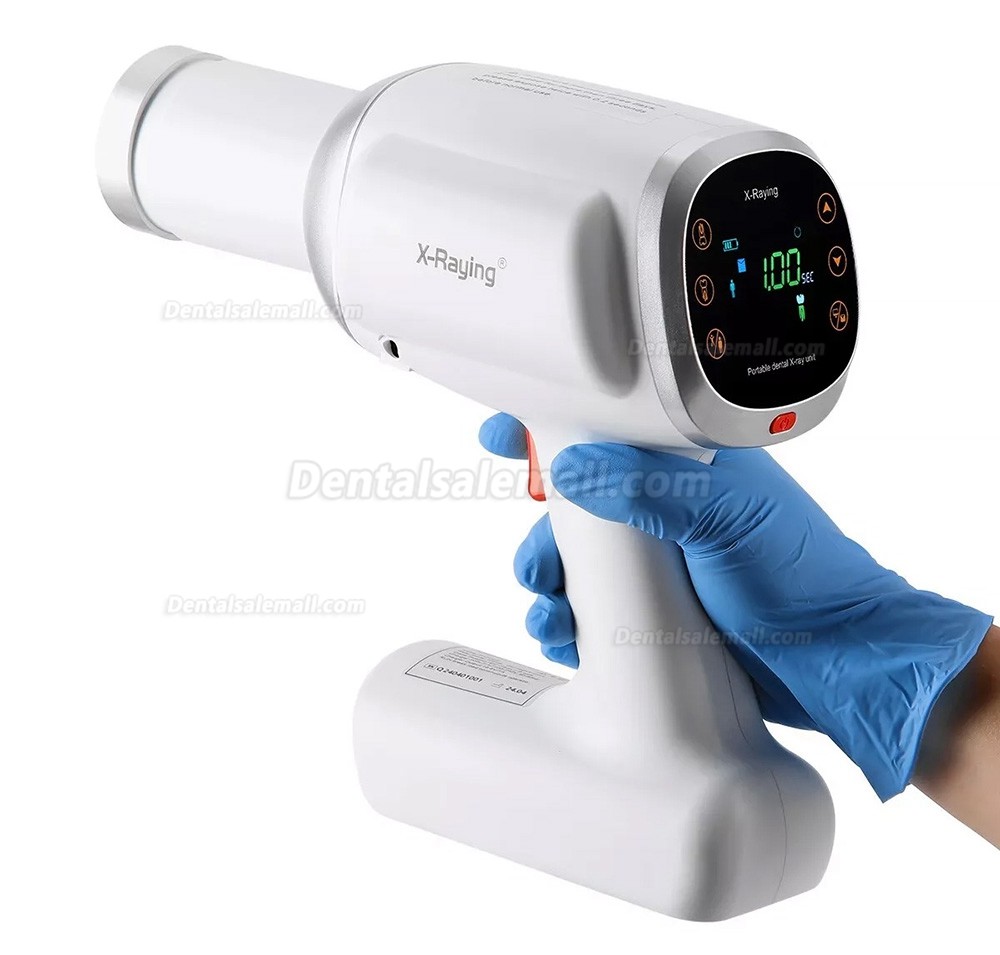
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Handheld X-Ray Machines
Frequently Asked Questions (FAQ) – Purchasing Handheld X-Ray Machines in 2026
As dental practices increasingly adopt portable imaging solutions for enhanced mobility and infection control, selecting the right handheld X-ray device is critical. Below are five essential questions and expert answers to guide clinics and distributors in making informed procurement decisions.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a handheld X-ray machine in 2026? | Most modern handheld X-ray devices in 2026 operate on low-voltage DC power (typically 12–24V), supplied via lithium-ion battery packs. These systems are designed for global compatibility and do not require direct high-voltage AC connections. However, ensure that the charging station or docking unit is compatible with local mains voltage (100–240V, 50/60Hz). Always verify input voltage specifications with the manufacturer, especially for clinics in regions with unstable power grids. |
| 2. Are spare parts readily available for handheld X-ray units, and what components commonly require replacement? | Yes, reputable manufacturers and distributors now maintain global spare parts inventories for key components. Commonly replaced parts include rechargeable battery packs, collimators, protective tube housings, control buttons, and wireless communication modules. In 2026, many suppliers offer modular designs to simplify field repairs. Distributors should confirm parts availability, lead times, and whether components are backward-compatible across model generations. |
| 3. Does installation of a handheld X-ray machine require professional setup or facility modifications? | Handheld X-ray units are designed for plug-and-play deployment and do not require structural modifications or fixed installations. However, regulatory compliance (e.g., radiation safety zoning, shielding assessments) may necessitate a site evaluation by a qualified medical physicist or radiation safety officer. The device itself only requires secure storage, a charging station, and integration with existing imaging software (e.g., DICOM compatibility). Manufacturer-certified technicians typically provide on-site training and system validation. |
| 4. What warranty coverage is standard for handheld X-ray machines in 2026, and what does it include? | As of 2026, most premium handheld X-ray systems come with a standard 3-year comprehensive warranty covering manufacturing defects, sensor performance, and internal electronics. Battery packs are typically covered for 1–2 years due to limited charge cycles. The warranty often includes labor, return shipping, and software updates. Extended warranties (up to 5 years) are available, especially for high-volume clinics and distributor service agreements. Always confirm exclusions such as physical damage, improper handling, or unauthorized repairs. |
| 5. How do voltage fluctuations or power surges impact handheld X-ray device longevity, and are surge protections built-in? | While the handheld unit itself runs on battery power and is isolated from grid fluctuations, the charging dock is susceptible to voltage spikes. Leading 2026 models incorporate multi-layer circuit protection, including over-voltage, over-current, and thermal safeguards in the charging module. For regions with unstable power, we recommend using an external surge protector or uninterruptible power supply (UPS). Prolonged exposure to power anomalies can degrade battery life and charging circuitry over time. |
Need a Quote for Hand Held X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


