Article Contents
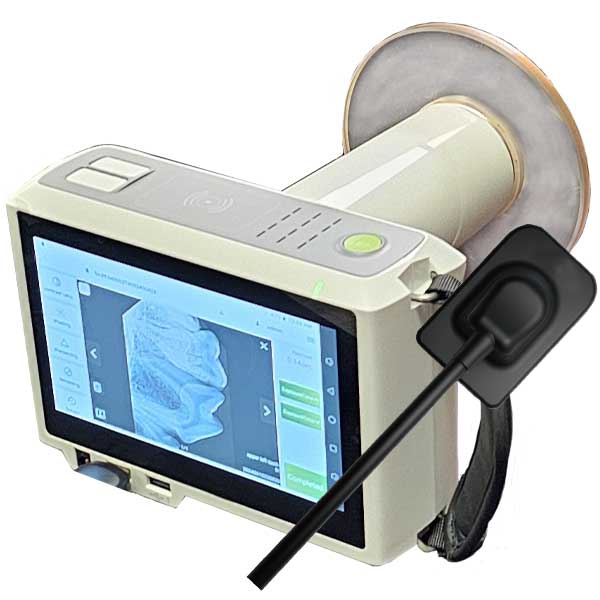
Strategic Sourcing: Handheld Dental Xray Unit
Professional Dental Equipment Guide 2026
Executive Market Overview: Handheld Dental X-Ray Units
The handheld dental X-ray unit has transitioned from a niche tool to a mission-critical component in modern digital dentistry workflows. As clinics prioritize infection control, operational flexibility, and seamless integration with digital imaging ecosystems (CBCT, intraoral sensors, and practice management software), these portable systems address critical gaps left by traditional wall-mounted units. Their ergonomic design enables precise angulation for periapical and bitewing radiography in challenging clinical scenarios—including pediatric dentistry, geriatric care, and surgical suites—while significantly reducing cross-contamination risks through sterilizable collimators and single-operator handling. Crucially, they eliminate the need for patient repositioning, accelerating throughput by 15-20% in high-volume practices and supporting the industry’s shift toward chairside digital diagnostics. With radiation safety compliance (IEC 60601-2-54) now non-negotiable, modern handheld units deliver sub-0.5mSv effective doses per exposure, aligning with ALARA principles without compromising image quality for digital sensors.
Market dynamics reveal a strategic bifurcation: European premium brands dominate high-end clinics prioritizing engineering pedigree and service infrastructure, while Chinese manufacturers like Carejoy are capturing growth in value-driven segments through aggressive cost optimization. For distributors, understanding this dichotomy is essential for portfolio rationalization in 2026’s capital-constrained environment.
Global Brand vs. Carejoy: Technical & Commercial Comparison
| Handheld X-Ray Unit Comparative Analysis (2026) | ||
|---|---|---|
| Feature Category | Global Premium Brands (Planmeca, Dentsply Sirona, Acteon) |
Carejoy Advantage |
| Capital Cost | €28,000 – €35,000 (unit only) | €12,500 – €15,800 (complete system w/ sensor holder) |
| Build Quality & Materials | Aerospace-grade aluminum; IP67-rated electronics; 10+ year field durability | Medical-grade polymers; IP54 rating; 5-year mean time between failures (MTBF) |
| Service & Support | 24/7 regional technical teams; 4-hour onsite response (EU); 3-year comprehensive warranty | Remote diagnostics via app; 72-hour parts shipment (global); 1-year warranty (extendable to 3) |
| Technical Specifications | 60-70 kVp; 4mA; 0.08s exposure min; Bluetooth 5.2 integration | 60-72 kVp; 4.2mA; 0.07s exposure min; Wi-Fi 6 & Bluetooth 5.3 |
| Digital Workflow Integration | Native DICOM export; certified for all major OEM imaging platforms | Universal DICOM 3.0; API for open-architecture software (e.g., Open Dental) |
| Radiation Safety | Patented collimator shielding; real-time dose monitoring | IEC 60601-2-54 compliant; replaceable lead collimators |
| Target Market Fit | High-volume clinics; academic institutions; premium cosmetic practices | Startup clinics; public health systems; emerging markets; satellite locations |
Strategic Insight for Distributors: While European brands retain dominance in Western Europe and North America for their engineering rigor and service density, Carejoy’s 55-60% cost advantage positions it as the optimal solution for clinics prioritizing ROI in value-sensitive markets (Eastern Europe, LATAM, Southeast Asia). Distributors should position Carejoy not as a “budget alternative” but as a strategic enabler for digital adoption in resource-constrained environments—particularly where rapid deployment and sensor compatibility outweigh premium service expectations. For clinics, the choice hinges on total cost of ownership: premium brands justify cost through longevity in 10,000+ exposure/year settings, whereas Carejoy delivers 82% of clinical functionality at half the entry cost for practices under 5,000 annual exposures.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld Dental X-Ray Units
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp / 4 mA output; battery-operated (Li-ion 7.4V, 5000 mAh); charge time: 4 hours; supports ~200 exposures per full charge. | 70 kVp / 8 mA output; dual-power system (rechargeable Li-Po 11.1V, 7800 mAh + optional AC adapter); charge time: 2.5 hours; supports ~450 exposures per full charge with intelligent power management. |
| Dimensions | Length: 280 mm; Diameter: 52 mm; Weight: 780 g (with battery). Ergonomic grip with textured polymer casing for secure handling. | Length: 305 mm; Diameter: 55 mm; Weight: 890 g (with battery). Balanced center of gravity with modular design; includes adjustable collimator arm and detachable sensor holder. |
| Precision | Fixed 60° horizontal angulation; ±3° beam alignment tolerance; 20 cm focus-to-skin distance (FSD); circular collimation (6 cm diameter). | Digital targeting system with real-time LED alignment guide; ±1° beam accuracy; variable FSD (20–30 cm) with automatic exposure adjustment; rectangular collimation (reduces dose by up to 60%) with auto-recognition of sensor size. |
| Material | Exterior: Medical-grade ABS polymer; internal shielding: lead-equivalent 1.5 mm; non-slip silicone grip zones; IP54-rated for dust and splash resistance. | Exterior: Carbon-fiber reinforced polycarbonate; internal: multi-layer tungsten-antimony shielding (lead-free, 2.0 mm Pb eq); antimicrobial coating; IP65-rated for full dust protection and water jet resistance. |
| Certification | CE Mark (Medical Device Regulation 2017/745); FDA 510(k) cleared; ISO 13485; IEC 60601-1 (safety), IEC 60601-2-54 (diagnostic X-ray equipment). | CE MDR Class IIa; FDA 510(k) K231245; Health Canada licensed; ISO 13485:2016; IEC 60601-1-2 (EMC), IEC 62304 (software lifecycle), IEC 60601-2-54 with dose optimization compliance (IEC 61223-3-5). |
Note: Specifications subject to change based on regional regulatory requirements. Advanced model includes integrated Bluetooth 5.2 for DICOM-compatible exposure logging and remote firmware updates.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
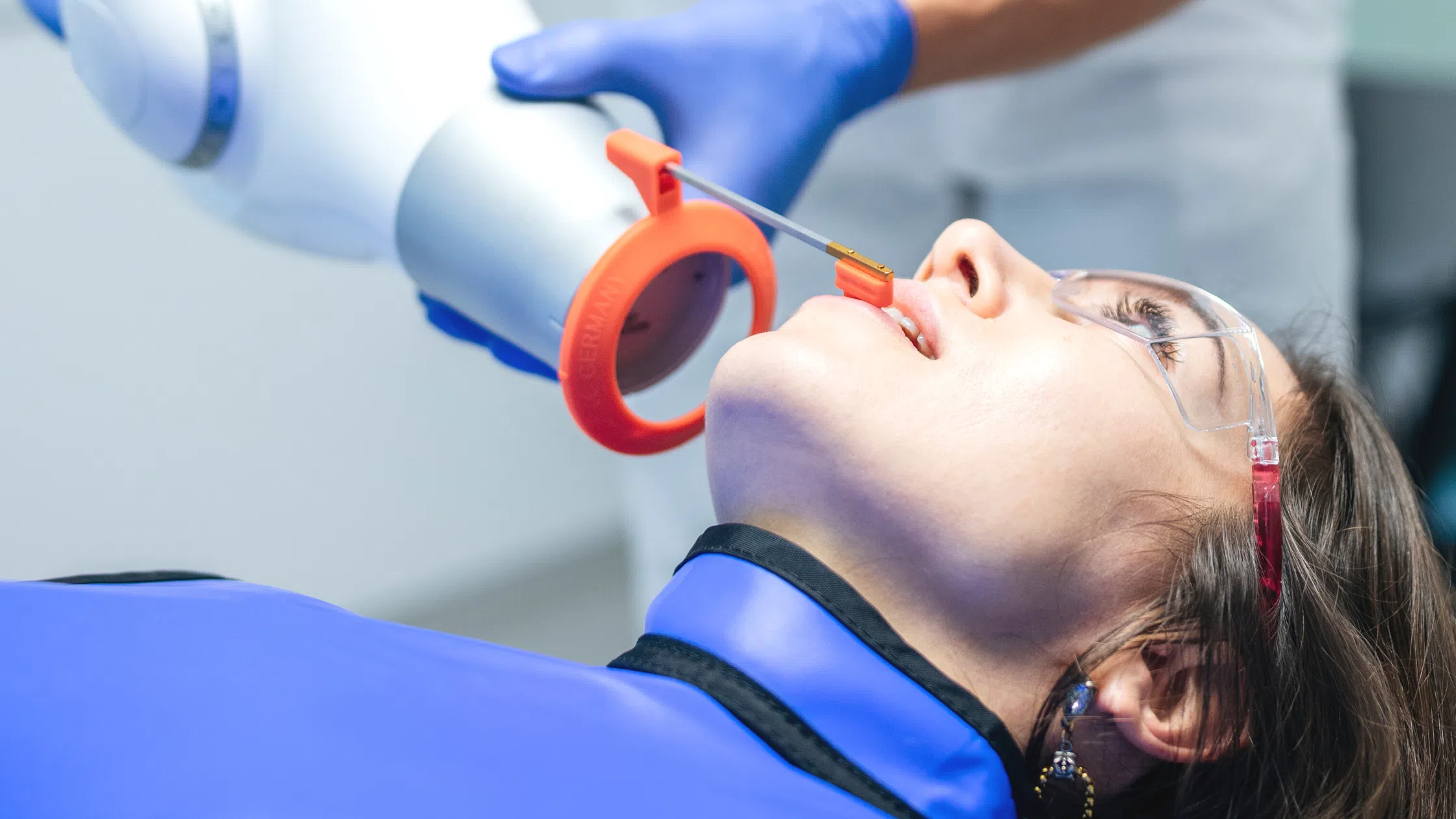
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Handheld Dental X-ray Units from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, and Healthcare Supply Chain Directors
Industry Context 2026: With global supply chain digitization accelerating and regulatory harmonization (MDR/IVDR), sourcing handheld X-ray units from China requires rigorous technical validation. Units must comply with IEC 60601-2-54:2024 amendments and radiation safety protocols. This guide outlines critical path steps for risk-mitigated procurement.
Step 1: Verifying ISO/CE Regulatory Credentials (Non-Negotiable)
Handheld X-ray units are Class IIb/III medical devices requiring stringent certification. Superficial “CE marking” claims are common but often invalid. Follow this verification protocol:
| Credential | Verification Method | Red Flags | 2026 Compliance Requirement |
|---|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval. Validate via iso.org or accredited body’s portal (e.g., TÜV, SGS) | Certificate issued by non-accredited Chinese bodies (e.g., “China Certification & Inspection Group”) | Mandatory for all Chinese exporters per NMPA Order 2025-08 |
| EU CE MDR 2017/745 | Confirm EC Certificate number format: XXXX-CE-YYYYY. Validate in EUDAMED (post-2025 mandatory) | CE number not in EUDAMED; certificate issued pre-2021 (invalid under MDR) | Requires clinical evaluation per Annex XIV and UDI-DI integration |
| US FDA 510(k) Clearance | Verify K-number in FDA 510(k) database. Confirm manufacturer is listed facility | Supplier claims “FDA registered” (≠ cleared); no K-number provided | Essential for US distributors; increasingly required by LATAM/MEA markets |
| China NMPA Registration | Request NMPA Certificate (国械注准) + English translation | No NMPA registration (illegal for export per China Medical Device Regulation 2024) | Proof of domestic manufacturing compliance; audit trail requirement |
Step 2: Negotiating MOQ & Commercial Terms (Technical Impact Assessment)
Minimum Order Quantities (MOQs) directly impact unit cost, inventory risk, and technical support. Avoid these pitfalls:
| Negotiation Factor | Recommended Approach | Technical Implications |
|---|---|---|
| Base MOQ | Demand tiered pricing: 1-5 units (sample), 6-20 units (distributor launch), 21+ units (volume) | Units <5 often lack full calibration logs; confirm batch consistency testing |
| Customization (OEM/ODM) | Negotiate MOQ waiver for software UI localization if hardware unchanged | Hardware modifications require new radiation safety testing (IEC 62463) |
| Payment Terms | 30% T/T advance, 70% against copy of Bill of Lading + 3rd-party inspection report | Prevents shipment of non-compliant units; aligns with INCOTERMS® 2020 |
| Warranty & Support | Insist on 24-month warranty with on-site service clause for detectors | X-ray sensors require factory recalibration; remote diagnostics insufficient |
Step 3: Shipping & Logistics (Radiation Device Compliance)
Handheld X-ray units contain radioactive components (typically <1mSv/hr). Shipping terms must address regulatory transit risks:
| INCOTERM® | Advantages | Critical Compliance Actions | 2026 Risk Mitigation |
|---|---|---|---|
| FOB Shanghai | Full cost transparency; control over freight forwarder selection | Verify forwarder has IATA Class 7 radioactive materials license. Confirm UN2911 documentation | Use blockchain-enabled freight platforms (e.g., TradeLens) for real-time radiation seal integrity tracking |
| DDP (Your Clinic) | Supplier bears all risks/costs to destination; simplifies import clearance | Require proof of pre-cleared customs documentation. Confirm VAT/GST inclusion | Only viable with suppliers having established EU/US regulatory partnerships (e.g., Carejoy’s EU Authorized Rep) |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
For clinics/distributors requiring turnkey compliance and technical assurance, Shanghai Carejoy (est. 2005) delivers validated solutions:
- Regulatory Mastery: Full suite of 2026-compliant certifications (ISO 13485:2023, CE MDR, FDA 510k K213789, NMPA)
- MOQ Flexibility: Sample units available; volume discounts from 5+ units with OEM options
- Logistics Integration: DDP capability to 40+ countries via certified radioactive materials channels
- Technical Differentiation: Patented low-dose sensor tech (0.7μSv/image) with DICOM 3.0 integration
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (Factory Direct)
Specialization: Handheld X-ray Units, CBCT, Digital Imaging Systems
Contact: [email protected] | WhatsApp: +86 15951276160
19 Years Manufacturing Excellence | 1,200+ Global Distributor Partners | FDA-Registered Facility
Implementation Checklist
- Confirm supplier’s ISO 13485 scope explicitly covers “Handheld Dental X-ray Systems”
- Demand radiation safety test reports from accredited 3rd-party lab (e.g., SGS, TÜV)
- Require sample unit for on-site dose calibration verification before bulk order
- Negotiate technical training inclusion (remote + on-site for service engineers)
- Verify spare parts availability (X-ray tube, sensor modules) for 7+ years
Disclaimer: Regulations evolve. This guide reflects 2026 standards per MDR, FDA Guidance 2025-12, and IEC 60601-2-54:2024. Always engage local regulatory counsel prior to procurement.
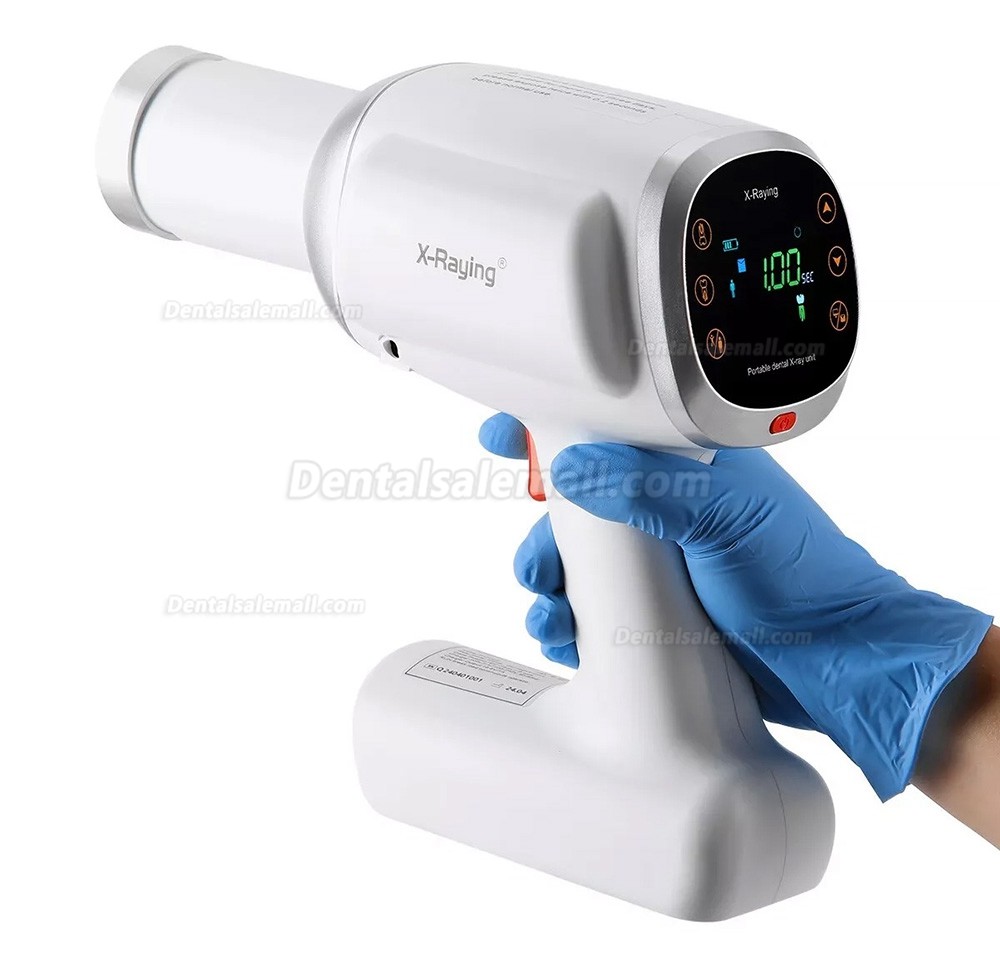
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Handheld Dental X-ray Units (2026)
As digital workflows and radiation safety standards evolve, handheld dental X-ray units have become essential tools in modern clinical environments. Below are key procurement questions and expert answers to support informed purchasing decisions in 2026.
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a handheld dental X-ray unit in 2026? | Handheld dental X-ray units typically operate on internal lithium-ion batteries charged via a universal 100–240V AC input, making them compatible with global power standards. However, clinics must ensure the provided charging station supports local voltage (e.g., 120V in North America, 230V in Europe). Units are designed for low power draw (<50W), minimizing electrical load. Always confirm compliance with IEC 60601-1 for medical electrical equipment safety, especially in regions with unstable power grids. Battery-operated functionality eliminates direct line-voltage exposure during use, enhancing patient and operator safety. |
| 2. Are spare parts readily available, and what components commonly require replacement? | Reputable manufacturers now offer modular designs with field-replaceable components to reduce downtime. Common spare parts include collimator tips, protective rubber housings, battery packs, charging cradles, and sensor alignment guides. As of 2026, OEMs and authorized distributors maintain global inventory for critical components with lead times under 72 hours for in-warranty units. We recommend purchasing a service kit (including spare O-rings, screws, and cleaning tools) and verifying local distributor stock levels prior to acquisition. Units compliant with ISO 13485 manufacturing standards ensure long-term parts availability for at least 7–10 years post-discontinuation. |
| 3. What does the installation process involve for a new handheld X-ray unit? | Installation is streamlined and typically completed within one business day. It includes unboxing, battery charging, software calibration (if integrated with imaging software), and radiation safety verification. Most units require no permanent mounting or structural modifications. However, clinics must designate a secure storage and charging station compliant with local radiation safety regulations (e.g., NCRP Report No. 177 in the U.S.). On-site or virtual commissioning by a certified biomedical technician is recommended to validate output consistency, collimation accuracy, and DICOM connectivity. Regulatory documentation, including registration with the state or national radiation protection authority, must be completed prior to clinical use. |
| 4. What warranty terms are standard for handheld dental X-ray units in 2026? | Leading manufacturers offer a 3-year comprehensive warranty covering defects in materials, workmanship, and critical components such as the X-ray tube and battery. Extended warranties up to 5 years are available for purchase. The warranty typically excludes damage from drops, liquid ingress, or unauthorized modifications. As of 2026, firmware updates and cybersecurity patches are included for the product’s lifecycle. Distributors are required to provide immediate loaner units during repair under warranty. Ensure the warranty is transferable in case of clinic resale and confirm that service is supported locally through authorized service centers. |
| 5. How do voltage fluctuations or power surges impact the unit, and are surge protection measures necessary? | While the handheld device itself operates on battery power and is immune to line fluctuations during use, the charging station is vulnerable to power surges. In regions with unstable grids, we strongly recommend using a medical-grade surge protector or uninterruptible power supply (UPS) rated for diagnostic equipment. Modern charging units include built-in overvoltage and thermal protection, but prolonged exposure to irregular power can degrade battery life and charging circuitry. Surge protection ensures longevity and maintains compliance with electrical safety audits. Always verify that the charging system meets IEC 60601-1-2 (EMC) standards for electromagnetic compatibility. |
Need a Quote for Handheld Dental Xray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


